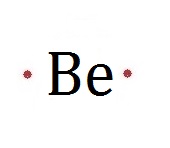Scientific Tutor
Archive for the ‘Chemistry’ Category
Chem – Naming Ionic Compounds Part 2
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
How do you use an ionic name to create the ionic compound?
Going in the other direction (taking the name and making the ionic compound), however, is a littler harder. First, you have to find the charges of the elements, and then you have to get them to total up to zero. How many of each element you need depends on how many each take to get to zero. In math, they call this concept the least common multiple.
Examples: Give the ionic compound from the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it. VIDEO Ionic Naming Examples 2.
| Lithium Phosphide | Li3P |
| Radium Iodide | RaI2 |
VIDEO Ionic Naming Demonstrated Example 2: Give the ionic compound from the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
Sodium Chloride
Step 1:
What is the symbol for Chloride?
Answer: Cl
| Cl | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the ion of Chloride?
Answer: -1
| Cl– | ||
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the symbol for Sodium?
Answer: Na
| Na | Cl– | |
| 1 | ||
| 1 | ||
| Total = |
Step 4:
What is the ion of Sodium?
Answer: +1
| Na+ | Cl– | |
| 1 | ||
| 1 | ||
| Total = |
Step 5:
What is the least common denominator between 1 and 1?
Answer: 1
| Na+ | Cl– | |
| 1 | ||
| 1 | ||
| Total = | +1 | -1 |
Step 6:
What is the complete chemical formula?
COMPLETE ANSWER: NaCl
VIDEO Ionic Naming Demonstrated Example 3: Give the ionic compound from the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
Calcium Phosphide
Step 1:
What is the symbol for phosphorous?
Answer: P
| P | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the charge for phosphorous?
Answer: -3
| P3- | ||
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the symbol for calcium?
Answer: Ca
| Ca | P3- | |
| 1 | ||
| 1 | ||
| Total = |
Step 4:
What is the charge for calcium?
Answer: +2
| Ca2+ | P3- | |
| 1 | ||
| 1 | ||
| Total = |
Step 5:
What is the least common denominator between 2 and 3?
Answer: 6
| Ca2+ | P3- | |
| 1 | ||
| 1 | ||
| Total = | +6 | -6 |
Step 6:
How many phosphorous do you need to add up to a total of -6?
Answer: You need 2 phosphorus
| Ca2+ | P3- | |
| 1 | P3- | |
| 1 | ||
| Total = | +6 | -6 |
Step 7:
How many calcium do you need to add up to a total of +6?
Answer: You need 3 Ca
| Ca2+ | P3- | |
| Ca2+ | P3- | |
| Ca2+ | ||
| Total = | +6 | -6 |
Step 8:
What is the complete chemical formula?
COMPLETE ANSWER: Ca3P2
PRACTICE PROBLEMS: Give the ionic compound from the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
| Lithium Chloride | LiCl |
| Calcium Iodide | CaI2 |
| Beryllium Oxide | BeO |
| Strontium Phosphide | St3P2 |
| Potassium Silicide | K4Si |
| Sodium Sulfide | Na2S |
Chem – Naming Ionic Compounds Part 1
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
How do you name ionic compounds?
For the ionic system of naming, it does not depend on how many of each element are in the compound. The key for the ionic system of naming is what the ion of each element is. The ions can be found on this periodic table. Any ionic compound should add up to a charge of zero unless otherwise stated.
Examples: Name the ionic compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
VIDEO Ionic Naming Examples 1.
| LiF | Lithium Fluoride |
| MgBr2 | Magnesium Bromide |
| Al2O3 | Aluminum Oxide |
| K2S | Potassium Sulfide |
VIDEO Ionic Naming Demonstrated Example 1: Name the ionic compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
BaF2
Step 1:
What is the name for the symbol Ba?
Answer: Barium
Step 2:
What is the name for the symbol F?
Answer: Fluorine
Step 3:
How do you modify the ending of Fluorine?
Answer: Becomes fluoride
Step 4:
What is the complete chemical name?
COMPLETE ANSWER: Barium Fluoride
PRACTICE PROBLEMS: Name the ionic compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
| NaI | Sodium Iodide |
| BeF2 | Beryllium Fluoride |
| Ba3P2 | Barium Phosphide |
| Rb3N | Rubidium Nitride |
| K2O | Potassium Oxide |
| CaCl2 | Calcium Chloride |
Chem – Naming Covalent Compounds Part 2
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
How do you use a covalent (molecular) name to create the covalent compound?
Now that we have taken the covalent chemical compound and produced the chemical name (as we did in the previous section) we should try the other direction. That is taking the chemical name and producing the chemical compound. Remember to use the covalent prefixes from the picture below.
Examples: Give the chemical formula from the name using a regular periodic table and the covalent prefixes. VIDEO Covalent Naming Examples 2.
| Triboron Tetracarbide | B3C4 |
| Nitrogen Trifluoride | NF3 |
| Heptasulfur Diselenide | S7Se2 |
| Bromine Monochloride | BrCl |
VIDEO Covalent (Molecular) Naming Demonstrated Example 2: Give the chemical formula from the name using a regular periodic table and the covalent prefixes.
Pentaphosphorus Dibromide
Step 1:
What does the prefix “penta” mean?
Answer: 5
Step 2:
What is the symbol for phosphorus?
Answer: P
Step 3:
How much phosphorus do you have?
Answer: 5
Step 4:
What does “di” mean?
Answer: 2
Step 5:
What element does bromide refer to?
Answer: Bromine
Step 6:
What is the symbol for bromine?
Answer: Br
Step 7:
How much bromine do you have?
Answer: 2
Step 8:
What is the chemical formula?
COMPLETE ANSWER: P5Br2
PRACTICE PROBLEMS: Give the chemical formula from the name using a regular periodic table and the covalent prefixes.
| Silicone Tetrahydride | SiH4 |
| Diarsenic Hexasulfide | As2S6 |
| Trinitrogen Heptaoxide | N3O7 |
| Octaphosphorus Nonaxenide | P8Xe9 |
Chem – Naming Covalent Compounds Part 1
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
How do you name covalent (molecular) compounds?
For the covalent system of naming, it depends on how many of each element there is in a compound. You describe the amount of each element by using a prefix word that indicates a number. The prefixes names for the system are as follows:
These prefixes are used in front of each elemental name in a compound. Sometimes the “a” on the end of the prefixes, like penta or octa, will be omitted. Don’t worry about it too much, right now.
Examples: Give the name of each compound using the covalent prefixes and a regular periodic table. VIDEO Covalent Naming Examples 1.
| C2O4 | Dicarbon Tetroxide |
| P3Br10 | Triphosphorus Decabromide |
One trick in this whole system has to do with the mono- prefix. Mono- is a special prefix that you do not always use. You have heard this exception before but may not have realized it. Take the compound CO2. Think… what is the name of it? Right, carbon dioxide. So why does carbon not have a mono- prefix on it? The exception I was talking about earlier is just that. If the first element in the compound is only one of that element, you do not use the mono- prefix. Here’s an additional example to drive the point home. Another common compound you have heard of is carbon monoxide. So what does it look like? That is right: CO. It is a perfect example of how the first element, carbon, has no prefix, but the second has a prefix. This rule is only true for the mono- prefix, so do not use it for any other purposes in covalent naming.
VIDEO Covalent (Molecular) Naming Demonstrated Example 1: Give the name of each compound using a regular periodic table and the covalent prefixes.
ClF2
Step 1:
What does Cl stand for?
Answer: Chlorine
Step 2:
How much chlorine do you have?
Answer: 1
Step 3:
What is the prefix for the chlorine?
Answer: It should be mono but since it is the first element you don’t write it.
Step 4:
What does F stand for?
Answer: Fluorine
Step 5:
How much fluorine do you have?
Answer: 2
Step 6:
What is the prefix for the fluorine?
Answer: Di
Step 7:
How do you modify the ending of fluorine?
Answer: Becomes fluoride
Step 8:
What is the complete chemical name?
COMPLETE ANSWER: Chlorine Difluoride
PRACTICE PROBLEMS: Give the name of each compound using a regular periodic table and the covalent prefixes.
| O2F2 | Dioxygen Difluoride |
| PCl3 | Phosphorous Trichloride |
| N4S5 | Tetranitrogen Pentasulfide |
| Se7I10 | Heptaselenium Deciodide |
| H2O | Dihydrogen Monoxide |
| BBr3 | Boron Tribromide |
Chem – Changing Ending of Name to IDE
How do you change the ending of a chemical name?
The second part of the naming system that both covalent and ionic compounds have in common is that any binary compound (compound made up of two elements) has the ending -IDE in the name. This -IDE usually replaces the -INE suffix in most elemental names. If you take the element fluorine, it becomes fluoride when it is part of a compound. Oxygen can be strange. It becomes oxide.
Examples: I will supply you with the beginning of the name and you give me the ending of the name. The periodic table link may be useful. VIDEO Changing Chemical Name Ending Examples 1.
| KI | Potassium Iodide |
| CaF2 | Calcium Flouride |
| Ba3N2 | Barium Nitride |
| SO2 | Sulfur Dioxide |
| Si2Br5 | Disilicon Pentabromide |
PRACTICE PROBLEMS: I will supply you with the beginning of the name and you give me the ending of the name. The periodic table link may be useful.
| LiCl | Lithium Chloride |
| SrBr2 | Strontium Bromide |
| Fr2Se | Francium Selenide |
| PO | Phosphorus Monoxide |
| KI | Potassium Iodide |
Chem – First Element in Chemical Name
Which element comes first in the compound or name?
Naming a chemical compound involves first identifying it as either a covalent or ionic compound. However, both covalent and ionic chemicals have a couple rules in common.
ONE is that both the chemical formulas and the chemical names (nomenclature) tend to start with the element furthest to the left on the periodic table.
TWO is that both can end their names with IDE. The IDE endings will be discussed and demonstrated in the next section changing ending of name to IDE.
Here we will show you how to predict the first element in any compound. For example, CsF is Cesium Fluoride. Another example is SiO2 which is Silicon Dioxide. That means if we pick out two random elements and put them together we want them to be in the correct order.
Examples: Give the element name that would appear first when you go to name the compound regular periodic table. VIDEO First Element in a Chemical Name Examples 1.
| C and O | Carbon |
| Na and P | Sodium |
| Se and Ca | Calcium |
| F and Sn | Tin |
| Fe and I | Iron |
| As and V | Vanadium |
PRACTICE PROBLEMS: Give the elemental name that would appear first when you go to name the compound using the regular periodic table.
| Mg and Br | Magnesium |
| Si and Ni | Nickle |
| Li and Br | Lithium |
| Te and Au | Gold |
| F and Sr | Strontium |
Chem – LESSON 7: Nomenclature
What is the lesson about?
Nomenclature means naming. So this lesson is all about naming different chemical compounds. There are different ways to name different combinations of elements so the rules can get confusing.
Why is it critical to understand?
That is because naming of compounds tends to be added to questions from other lessons. Nomenclature can also come in handy when we are buying food products or medicines. Most chemicals in food and drugs are labeled according to the nomenclature system here.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to sections on Ions, Covalent Ionic and Metallic Bonds, Introduction to Polyatomic Ions, Identifying Polyatomic Ions in Compounds, Forming Ionic Compounds, and Breaking Apart Ionic Compounds.
New Learning Sections:
—> First Element in Chemical Name
—> Changing Ending of name to IDE
—> Naming Covalent Compounds Part 1
—> Naming Covalent Compounds Part 2
—> Naming Ionic Compounds Part 1
—> Naming Ionic Compounds Part 2
—> Ionic Compounds with Transition Metals Part 1
—> Ionic Compounds with Transition Metals Part 2
—> Ionic Compounds with Polyatomic Ions Part 1
—> Ionic Compounds with Polyatomic Ions Part 2
Other Nomenclature Areas:
I STRONGLY BELIEVE THAT ALL TEACHERS SHOULD NOT REQUIRE THESE OTHER AREAS TO BE COVERED ALONG SIDE THE ABOVE SECTIONS. However, some teachers insist you to learn them early in chemistry so I put the links below in case you needed them all in one place. They are integrated sections in other lessons on this website.
LINKS NOT IN / PAGES NOT COMPLETE YET
—> Naming Acids
—> Naming Organic Compounds
References Pages:
—> Metal / Non-Metal Periodic Table
Worksheets:
—> Nomenclature Worksheet 1 WITH ANSWERS
Chem – Breaking Apart Ionic Compounds
What sections should I know before attempting to learn this section?
—> Representation of Compounds and Molecules with Subscripts
—> Covalent, Ionic, and Metallic Bonds
—> Introduction to Polyatomic Ions Part 1
How do you break apart ionic compounds?
As we go forward into the future of learning chemistry it will become essential for us to be able to take ionic compounds and break them up into their component ions. It is a mindset that you have to develop as you go but right here we want to give you a boost or head start on it. All ionic compounds break up into positive ions (cations) and negative ions (anions). The positive ions (usually metals) are on the left side of the chemical formula and the negative ions (usually non-metals) are on the right side of the chemical formula.
Examples: Separate the CHEMICAL FORMULAS into their ionic components. Give the POSITIVE ion and the NEGATIVE ion and tell how many of each are in the CHEMICAL FORMULA. Use the ionic periodic table or the polyatomic ions list if needed. VIDEO Breaking Apart Ionic Compounds Examples 1.
| Formula | Positive | Negative |
| LiF | 1 Li1+ | 1 F1- |
| Al2O3 | 2 Al3+ | 3 O2- |
| HCN | 1 H1+ | CN1- |
| Mg(NO3)2 | 1 Mg2+ | 2 NO31- |
| Rb2Se | 2 Rb1+ | 1 Se2- |
| NH4OH | 1 NH41+ | 1 OH1- |
| Mn3N4 | 3 Mn4+ | 4 N3- |
ADD ONE DEMONSTRATED EXAMPLE
PRACTICE PROBLEMS: Pick out the POLYATOMIC IONS from the chemical formula. There may be ONE, TWO, or NONE. Use the ionic periodic table or the polyatomic ions list if needed.
| Formula | Positive | Negative |
| CsBr | 1 Cs1+ | 1 Br1- |
| Be3N2 | 3 Be2+ | 2 N3- |
| KNO3 | 1 K1+ | 1 NO31- |
| Ca(OH)2 | 1 Ca2+ | 2 OH1- |
| Ba3(PO4)2 | 3 Ba2+ | 2 PO43- |
| Ti2S | 2 Ti1+ | 1 S2- |
| CrSO4 | 1 Cr2+ | 1 SO42- |
Chem – Forming Ionic Compounds
What sections should I know before attempting to learn this section?
—> Representation of Compounds and Molecules with Subscripts
—> Introduction to Polyatomic Ions Part 1
—> Covalent, Ionic, and Metallic Bonds
How do you form ionic compounds?
The formation of ionic compounds comes from the ion rules of the periodic table. The first and most important rule of forming compounds is that they have to add up to a charge of zero unless otherwise stated. If we put two ions together, one positive and one negative, then we have to add as many elements to each side until we get a sum of zero. In math they call this the smallest common multiple. You can usually do this in chemistry by multiplying together the two numbers you are dealing with.
Examples: Put these two elements together to form an ionic compound.
| K and Br | KBr |
| Na and O | Na2O |
| Mg and N | Mg3N2 |
VIDEO Forming Ionic Compounds Demonstrated Example 1: Put these two elements together to form an ionic compound: Na and S
Step 1:
What is the charge on Na?
Answer: +1
| Na+ | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the charge on S?
Answer: -2
| Na+ | S2- | |
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the least common multiple between 1 and 2?
Answer: 2
| Na+ | S2- | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 4:
How much Na do you need to add up to +2?
Answer: 2 Na
| Na+ | S2- | |
| Na+ | ||
| 1 | ||
| Total = | +2 | -2 |
Step 5:
How much S do you need to add up to -2?
Answer: 1 S
| Na+ | S2- | |
| Na+ | ||
| 1 | ||
| Total = | +2 | -2 |
Step 6:
If you have 2 Na and 1 S what does the compound look like?
COMPLETE ANSWER: Na2S
VIDEO Forming Ionic Compounds Demonstrated Example 2: Put these two elements together to form an ionic compound: Ba and P
Step 1:
What is the charge on Ba?
Answer: +2
| Ba2+ | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the charge on P?
Answer: -3
| Ba2+ | P3- | |
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the least common multiple between 2 and 3?
Answer: 6
| Ba2+ | P3- | |
| 1 | ||
| 1 | ||
| Total = | +6 | -6 |
Step 4:
How much Ba do you need to add up to +6?
Answer: 3 Ba
| Ba2+ | P3- | |
| Ba2+ | ||
| Ba2+ | ||
| Total = | +6 | -6 |
Step 5:
How much P do you need to add up to -6?
Answer: 2 P
| Ba2+ | P3- | |
| Ba2+ | P3- | |
| Ba2+ | ||
| Total = | +6 | -6 |
Step 6:
If you need 3 Ba and 2 P what does the compound look like?
COMPLETE ANSWER: Ba3P2
VIDEO Forming Ionic Compounds Demonstrated Example 3: Put these two ions together to form an ionic compound: Mg2+ and OH–
Step 1:
What is the charge on Mg?
Answer: +2
| Mg2+ | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the charge on OH–?
Answer: -3
| Mg2+ | OH– | |
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the least common multiple between 2 and 1?
Answer: 2
| Mg2+ | OH– | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 4:
How much Mg do you need to add up to +2?
Answer: 1
| Mg2+ | OH– | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 5:
How much OH do you need to add up to -2?
Answer: 2
| Mg2+ | OH– | |
| OH– | ||
| 1 | ||
| Total = | +2 | -2 |
Step 6:
If you need 1 Mg and 2 OH what does the compound look like?
COMPLETE ANSWER: Mg(OH)2
PRACTICE PROBLEMS: Put these two elements or ions together to form an ionic compound. Don’t Forget to use the ion rules of the periodic table, if you need them.
| Li and O | Li2O |
| Al and P | AlP |
| Cs and N | Cs3N |
| Ca and I | CaI2 |
| Ra and As | Ra3As2 |
| Mg and C | Mg2C |
| NH4+ and Te2- | (NH4)2Te |
| Ba2+ and CN– | Ba(CN)2 |
| Be2+ and PO43- | Be3(PO4)2 |
Chem – Identifying Polyatomic Ions in Compounds
How do you learn to find polyatomic ions in compounds?
What you want to start doing in this section is getting good at spotting polyatomic ions whenever and wherever you see them. Many of them will be hidden in the chemical formulas you will see in the future of chemistry. The better you become at identifying them, the better you will be at solving future chemistry problems.
Examples: Pick out the POLYATOMIC IONS from the chemical formula. There may be ONE, TWO, or NONE. Use the polyatomic ions list if needed.VIDEO Identifying Polyatomic Ions in Compounds Example 1.
| Formula | Polyatomic Ions |
| Mg(NO3)2 | NO31- |
| HCN | CN1- |
| CaBr2 | NONE |
| NH4OH | NH41+ OH1- |
| (H3O)2CO3 | H3O1+ CO32- |
PRACTICE PROBLEMS: Pick out the POLYATOMIC IONS from the chemical formula. There may be ONE, TWO, or NONE. Use the polyatomic ions list if needed.
| Formula | Polyatomic Ions |
| Fe3(PO4)2 | PO43- |
| NaC2H3O2 | C2H3O21- |
| NH4CN | NH41+ CN1- |
| K2S | NONE |
| BeSO4 | SO42- |
Chem – Memorization of Polyatomic Ion Charges
How do you Better and More Quickly Memorize Polyatomic Ion Charges?
VIDEO Explanation of Tips to Memorize Polyatomic Ions
For many of the polyatomic ions on the list even though their names change like ATE, ITE, PER, HYPO, the charge of their ion does not change.
Examples:
| Sulfate | SO42- |
| Sulfite | SO32- |
| 1 | |
| Nitrate | NO31- |
| Nitrite | NO21- |
PRACTICE PROBLEMS: Without looking at the polyatomic ions list try to write in the missing charge. The first problem is done for you as an example.
| Name | Charge |
| 1 | |
| Hypochlorite | -1 |
| Chlorate | -1 |
| 1 | |
| Carbonate | -2 |
| Carbonite | -2 |
| 1 | |
| Hypoiodite | -1 |
| Periodate | -1 |
| 1 | |
| Phosphate | -3 |
| Phosphite | -3 |
When you finish the lesson, try out the worksheet on polyatomic ions.
Chem – PER Versus HYPO
What does PER Versus HYPO mean in the Beginning of Polyatomic Ion Names?
Video Explanation of PER Versus HYPO in the Beginning of Polyatomic Ion Names
Group 2 of the polyatomic ion list starts introducing us to the addition of PER and HYPO. Like ATE and ITE, PER and HYPO are a continuation of the counting of oxygens. In most cases (but not all) PER adds one more oxygen from ATE. In most cases (but not all) HYPO is minus one oxygen from ITE.
Examples: Adding Per or Hypo can changed the count of the oxygens.
| IO4– | Periodate |
| IO3– | Iodate |
| 1 | |
| IO2– | Iodite |
| IO– | Hypoiodite |
PRACTICE PROBLEMS: Without looking at the polyatomic ions list try to write in how many oxygen each ion has by comparing it to its neighbor above or below. The first problem is done for you as an example.
| Name | Amount of Oxygen |
| 1 | |
| Perchlorate | 4 oxygen |
| Chlorate | 3 oxygen |
| 1 | |
| Chlorite | 2 oxygen |
| Hypochlorite | 1 oxygen |
| 1 | |
| Bromite | 2 oxygen |
| Hypobromite | 1 oxygen |
| 1 | |
| Perbromate | 4 oxygen |
| Bromate | 3 oxygen |
Chem – ATE Versus ITE
What does ATE Versus ITE mean on the end of Polyatomic Ion Names?
VIDEO Explanation of ATE Versus ITE on Polyatomic Ion Names
In Group 1 and 2 of the polyatomic ions list we can notice that many of the polyatomic ions have a name ending in -ATE or -ITE. The -ATE or -ITE is telling the reader each ion has certain a number of oxygens. To be clear, it does not exactly tell you how many oxygens, but it gives you an idea about them. Its purpose is to give you a consistent comparison between -ATE and -ITE.
Examples: A difference of one oxygen between them.
Carbonate has three oxygens
Carbonite has two oxygens
The hard rule here is -ate always has one more oxygen than -ite. Look at how each -ate and -ite are organized so that you could compare easily. However, -ate DOES NOT always mean it has three oxygens and the ending -ite does not always mean it has two oxygens.
PRACTICE PROBLEMS: Without looking at the polyatomic ions list try to write in how many oxygens each ion has by comparing it to its neighbor above or below. The first problem is done for you as an example.
| Name | Amount of Oxygen |
| 1 | |
| Sulfate | 4 oxygens |
| Sulfite | 3 oxygens |
| 1 | |
| Chlorate | 3 oxygens |
| Chlorite | 2 oxygens |
| 1 | |
| Nitrite | 2 oxygens |
| Nitrate | 3 oxygens |
| 1 | |
| Phosphite | 3 oxygens |
| Phosphate | 4 oxygens |
| 1 | |
| Carbonite | 2 oxygens |
| Carbonate | 3 oxygens |
| 1 | |
| Bromate | 3 oxygens |
| Bromite | 2 oxygens |
Chem – Polyatomic Ion Two Part Name
What is the Polyatomic Ion Two Part Naming System?
VIDEO Explanation of the Two Part Polyatomic Ion Naming System
First, open up the polyatomic ion list in this link and have it ready to look at as you are reading or viewing this section. This way you can look at the examples in the different groups as they are talked about. The first and most obvious thing I would like to point out is that, in most of the polyatomic ions, the first element in the polyatomic ion is what the name of the polyatomic ion starts with.
Examples:
Carbonate (CO32-) has carbon as its first element.
Nitrite (NO21-) has nitrogen as its first element.
The name is also split into two parts.
Examples:
| Part 1 | Part 2 |
| Carbon | ate |
| Nitr | ite |
PRACTICE PROBLEMS: Without looking at the polyatomic ions list try to fill in the first half of the name of the ions below. The first problem is done for you as an example.
|
Ion |
First Half of Name |
|
NO31- |
Nitr |
|
PO43- |
Phosph |
|
SO32- |
Sulf |
Chem – Introduction to Polyatomic Ions
What are polyatomic ions?
In the previous section of ions we looked at how ions could be created from a single atom of a single element. There are, however, ions that are made up of many different atoms bonded together. These are what we call the polyatomic ions. If you break down the word, poly means “many” and atomic means “atoms”. I designed several of the sections after this one to help you understand the polyatomic ions better. The polyatomic ion sections also help you find tricks to more easy memorize the polyatomic ions if needed.
Why are polyatomic ions important?
We interact with polyatomic ions all the time without realizing it. They are in the soil and streams and they are critical components to all life forms. Some of them are the nutrients that make up the components of all living things body. For example, phosphate is critical for farmers to spread in the field as part of fertilizer. At the same time phosphate makes up part of our blood, our bones, and our DNA.
Do I need to memorize the polyatomic ions?
YOU CAN LOOK AT THE POLYATOMIC ION LIST IN THE PICTURE BELOW OR IN THIS LINK. Whether or not you need to memorize the polyatomic ions will depend mostly on what kind of chemistry class you are in. In most general chemistry high school classes, you do not need to memorize them. However, honors high school classes may make you memorize a small number of these polyatomic ions but AP and college classes will make you memorize most of them.
What do I need to know or memorize about the polyatomic ions?
What you have to know is the name, chemical formula, and charge of everything in the polyatomic ion list below. I ranked the following polyatomic ions into groups. Group 1 you absolutely have to know how to spot or memorize and you will see them very often. Group 2 would be useful to know, but not critical. Some of the Group 3 polyatomic ions will be used in AP and college students. While you are reading through and learning this chapter you should always have the polyatomic ions link open so you can look back at it. All other sections in this lesson are just to help you understand the names of the polyatomic ions and to help you memorize them. If you know how to memorize them on your own then you don’t need to look at any more sections. However, I strongly suggest that you do continue with the rest of the sections.
Chem – Covalent, Ionic, and Metallic Bonds (Intramolecular Forces)
What is the difference between covalent, ionic, and metallic bonds?
Two or more elements are held together by their electrons, but the electrons can do this in different ways. Electrons are like the skin of the atom because they are on the outer parts of the atom. Atoms interact with each other mostly by contact or trading of electrons, just like people interact with each other by contacting skin in the form of a handshake. As you read through the explanations of the different types of bonds have the periodic table of metal versus non-metal elements open. If you are confused by this section then it is suggested you go back to the metals and non-metals section.
Covalent: One way to create a bond between elements is if those elements share electrons. The covalent bonds form between two or more NON-METAL elements. These bonds are typically strong and flexible. One good example of covalent bonds is the bonds in your skin. Your skin can be twisted to show its flexibility and it takes a very sharp edge, like a knife, to cut it, which shows its strength.
Ionic: Another way to create bond is if those elements steal electrons from each other. The ionic bonds form between at least one METAL and at least one NON-METAL element. These bonds are typically strong and brittle. One good example is salt. You can crush it with a hammer, which shows its strength. When that happens, it will crumble into smaller pieces showing its’ brittle nature.
Metallic: The third and least common type of bond forms if the elements create a sea of electrons. This means the electrons become delocalized from their original atom and flow easily from one atom to another. It is like if you were swimming in a lake or ocean. Your sweat delocalizes from your body and mixes in with all the other water around you. The metallic bonds form between two or more METALS. These bonds are typically strong, have moderate flexibility and conduct electricity well.
These 3 types of bonds (covalent, ionic, and metallic) make up what are called intramolecular bonds (Notice the first 5 letters: INTRAmolecular bonds). They are the bonds formed within one compound or molecule.
With this information we want to be able to identify different compounds that we come across. Any time we see a chemical in front of us we want to be able to label it as covalent, ionic, or metallic. The first stage of this is to be able to take two elements together and say what kind of bond would form between them? Examples are below.
Examples: Label each pair of elements as covalent, ionic, or metallic in terms of the bonds using the metals versus non-metals periodic table. VIDEO Explanation of bonding examples 1.
| Na to Br | Ionic |
| N to O | Covalent |
| Ni to Zn | Metallic |
| Ca to S | Ionic |
| Si to Cl | Covalent |
| F to F | Covalent |
| Sr to Cr | Metallic |
PRACTICE PROBLEMS: Label each pair of elements as covalent, ionic, or metallic in terms of the bonds bonds using the metals versus non-metals periodic table.
| Ba to P | Ionic |
| Li to C | Ionic |
| Cu to Pb | Metallic |
| Al to H | Ionic |
| I to Kr | Covalent |
| Fe to O | Ionic |
| Pt to Ba | Metallic |
| B to H | Covalent |
You also want to be able to look at complete compounds and be able to tell if they are covalent, ionic, or metallic.
Examples: Label each compound as covalent, ionic, or metallic in terms of the bonds using the metals versus non-metals periodic table. VIDEO Explanation of bonding examples 2.
| BeO | Ionic |
| MnAg2 | Metallic |
| SCl2 | Covalent |
| W2Se | Ionic |
| SiF4 | Covalent |
| CaSn | Metallic |
| AsBr2 | Covalent |
PRACTICE PROBLEMS: Label each compound as covalent, ionic, or metallic in terms of the bonds using the metals versus non-metals periodic table.
| BH3 | Covalent |
| CrF2 | Ionic |
| TeI2 | Covalent |
| Hg2Co | Metallic |
| Fr2S | Ionic |
| KAg | Metallic |
| N2O3 | Covalent |
How different compounds and molecules form the way they do depends on whether they are covalent, ionic, or metallic. Covalent will be discussed when you get to the valence electron dot structures lesson (Lewis structures lesson) later in the website. Ionic will be discussed in another section of this lesson called how to form ionic compounds. Metallic will more than likely not be discussed on this website.
Chem – Calculating the Molar Mass of Compounds
How do you calculate the molar mass?
After we have learned how to form these ionic compounds, we can move on to calculating the mass of a compound. This is what teachers will call a molar mass, or atomic mass, or molar weight, or an atomic weight. Most chemistry teachers will use all of those phrases in the previous sentence interchangeably. As far as you need to know, the molar mass, atomic mass, molar weight, or atomic weight are all talking about the same thing and you use the same resource to answer these problems. That resource is the periodic table. At the bottom of each individual element box on the periodic table will be the molar mass of each element. The way to solve the problems is to total up all the masses of the individual atoms. In order to keep the numbers simple and easy to read, I will usually round to the nearest whole number. If you are confused as to what the parenthesis mean then look back to a previous section representing compounds and molecules with subscripts.
Examples: Give the molar mass of the compounds or molecules.
| H2O | 18 g/mol |
| CO2 | 44 g/mol |
| N4S3 | 152 g/mol |
| (H3O)3As | 130 g/mol |
| Ra(BrO3)2 | 482 g/mol |
VIDEO Calculating Molar Mass Demonstrated Example 1: Give the molar mass of the compound or molecule: Al2S3
Step 1:
What is the mass of a single Aluminum?
Answer: 27 g/mol
Step 2:
How much Aluminum do we have?
Answer: 2
Step 3:
What is the total mass of the Aluminum?
Answer: 2 * 27 g/mol = 54 g/mol
Step 4:
What is the mass of a single Sulfur?
Answer: 32 g/mol
Step 5:
How much Sulfur do you have?
Answer: 3
Step 6:
What is the total mass of the Sulfur?
Answer: 3 * 32 g/mol = 96 g/mol
Step 7:
What is the total mass of the compound?
96 g/mol + 54 g/mol = 150 g/mol
COMPLETE ANSWER: 150 g/mol
VIDEO Calculating Molar Mass Demonstrated Example 2: Give the molar mass of the compound or molecule: Be(NO3)2
Step 1:
What is the mass of a single Beryllium?
Answer: 9 g/mol
Step 2:
How much Beryllium do we have?
Answer: 1
Step 3:
What is the total mass of the Beryllium?
Answer: 1 * 9 g/mol = 9 g/mol
Step 4:
What is the mass of a single Nitrogen?
Answer: 14 g/mol
Step 5:
How much Nitrogen do you have?
Answer: 2
Step 6:
What is the total mass of the Nitrogen?
Answer: 2 * 14 g/mol = 28 g/mol
Step 7:
What is the mass of a single Oxygen?
Answer: 16 g/mol
Step 8:
How much Oxygen do we have?
Answer: 6
Step 9:
What is the total mass of the Oxygen?
Answer: 6 * 16 g/mol = 96 g/mol
Step 10:
What is the total mass of the compound?
96 g/mol + 28 g/mol + 9 g/mol = 133 g/mol
COMPLETE ANSWER: 133 g/mol
PRACTICE PROBLEMS: Give the molar mass of the compounds or molecules. Use the periodic table.
| BaSe | 216 g/mol |
| Si5As8 | 740 g/mol |
| K3P | 131 g/mol |
| CH4 | 16 g/mol |
| C6H12O6 | 180 g/mol |
| BClF2 | 84 g/mol |
| (NH4)3P | 85 g/mol |
| Pb(Cr2O7)2 | 639 g/mol |
| Ba(C2H3O2)2 | 255 g/mol |
We will be reviewing the molar mass continuously throughout the first half of chemistry, so make sure you know how to solve the above problems and keep the techniques in mind.
Chem – Atoms, Molecules, and Compounds
What is the difference between atoms, molecules, and compounds?
Definitions are important to address in this lesson. At this point in chemistry, many people get confused by the words atom, molecule, and compound. An atom is the smallest indivisible part of matter. When you look at a chemical like Rb2S, 1 Rb is 1 atom. An atom is simply any single element written. So if a single element is written alone like P then it is only an atom. However, that P is also a molecule because it is separate from other chemicals. A molecule is simply any arrangement of atoms that are bound to each other but separate from other chemicals. I like to think of a molecule as a team. Most of the time a team consists of 2 or more people but in theory a team could be only one person. So most of the time a molecule consists of many different atoms, but it is possible for a molecule to consist of only 1 atom. The last definition we need to know is a compound. A compound is when two or more different elements are bound together. Our original example Rb2S is a compound. Compounds will be by far the most common chemical arrangement you will see.
Examples: Label whether each is a compound, molecule, or atom. You can give more than one answer if needed. VIDEO Atoms, Molecules, and Compounds Examples Video 1.
| NH3 | Compound and Molecule |
| He | Molecule and Atom |
| Br2 | Molecule |
| Xe | Molecule and Atom |
| Si4 | Molecule |
| Li2S | Compound and Molecule |
PRACTICE PROBLEMS: Label whether each is a compound, molecule, or atom. You can give more than one answer if needed.
| NaCl | Compound and Molecule |
| CO2 | Compound and Molecule |
| F2 | Molecule |
| S8 | Molecule |
| Ar | Molecule and Atom |
| C2H6O2 | Compound and Molecule |
Chem – Representation of Compounds and Molecules with Subscripts
Before we discuss the formation of any compounds, we have to make sure we have an understanding of how those compounds are represented in chemistry. In any type of chemistry class, the main focus of how many elements you have in a compound is what they call the subscripts. Subscripts are numbers that are placed to the right of and down towards the bottom of an element. The subscripts are a number that multiplies the element they are next to. If there is no subscript present then that means it is counted as a 1.
Examples: Identify what the subscripts of each element in the compounds below.
| K2O | K is 2 and O is 1 |
| SrF2 | Sr is 1 and F is 2 |
| Mg3P2 | Mg is 3 and P is 2 |
Some chemical representations also have parenthesis in them. These parenthesis are much like the ones you use in math class. That is parenthesis are used to multiply everything inside them by a number that is put outside of them.
Examples: How many atoms of each element are present in the following compounds.
| (NH4)2S | 2 N and 8 H and 1 S |
| Be(NO3)2 | 1 Be and 2 N and 6 O |
| Fe2(S2O3)3 | 2 Fe and 6 S and 9 O |
VIDEO Counting Compound Subscripts Demonstrated Example 1: How many atoms of each element are present in the compound below.
Ca3(PO4)2
Step 1:
What is the first element?
Answer: Ca
Step 2:
What is the subscript of the first element?
Answer: 3
Step 3:
Is Ca in parenthesis?
Answer: No
Step 4:
How many Ca atoms do we have?
Answer: 3 Ca
Step 5:
What is the next element?
Answer: P
Step 6:
What is the subscript of P?
Answer: 1
Step 7:
Is P in parenthesis?
Answer: Yes
Step 8:
What is the subscript of the parenthesis?
Answer: 2
Step 9:
How many P atoms do we have?
Answer: 1 * 2 = 2 P
Step 10:
What is the last element?
Answer: O
Step 11:
What is the subscript of O?
Answer: 4
Step 12:
Is O in parenthesis?
Answer: Yes
Step 13:
What is the subscript of the parenthesis?
Answer: 2
Step 14:
How many O atoms do we have?
Answer: 4 * 2 = 8 O
Step 15:
COMPLETE ANSWER: 3 Ca and 2 P and 8 O
PRACTICE PROBLEMS: How much of each element are present in the following compounds.
| LiCl | 1 Li and 1 Cl |
| Rb2S | 2 Rb and 1 S |
| Al2Se3 | 2 Al and 3 Se |
| CH4 | 1 C and 4 H |
| Cs2O | 2 Cs and 1 O |
| Cr3P2 | 3 Cr and 2 P |
| Sr(OH)2 | 1 Sr and 2 O and 2 H |
| Cr2(SO3)3 | 2 Cr and 3 S and 9 O |
| Pb3(PO4)4 | 3 Pb and 4 P and 16 O |
Chem – LESSON 6: Compounds and Bonding
What is this lesson about?
This lesson is mostly about how different elements interact to form different compounds. You will also learn how to represent different elements together in one compound and some of the measurements you can take with those compounds.
Why is it critical to understand?
If you are not able to understand how different elements come together and how those elements and compounds are recognized, then you will not be able to continue with lessons like nomenclature, valene electron dot structures (Lewis structures), and chemical equations. Beyond that, knowing how elements are put together also allows you to know how to take them apart. Whether you can dissolve them in water or whether you need to burn them in a fire. The type of the bonding that occurs also give you clues on how strong that bond is and therefore, what that material you have created can be used for.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to the section on Ions.
New Learning Sections:
—> Representation of Compounds and Molecules with Subscripts
—> Atoms, Molecules, and Compounds
—> Calculating the Molar Mass of Compounds
—> Covalent, Ionic, and Metallic Bonds
—> Introduction to Polyatomic Ions Part 1
—> Two Part Name for Polyatomic Ions Part 2
—> ATE versus ITE in Polyatomic Ion Names Part 3
—> PER versus HYPO in Polyatomic Ion Names Part 4
—> Memorizing Polyatomic ion Charges Part 5
—> Identifying Polyatomic Ions in Compounds Part 6
—> Breaking Apart Ionic Compounds
Reference Pages:
—> Ion Rules of the Periodic Table
Worksheets:
—> Compounds and Bonding Worksheet 1
—> Compounds and Bonding Worksheet 1 WITH ANSWERS
—> Polyatomic Ions Worksheet 1
—> Polyatomic Ions Worksheet 1 WITH ANSWERS
—> College: Polyatomic Ions Worksheet 2
—> College: Polyatomic Ions Worksheet 2 WITH ANSWERS
Chem – Calculating the Energy of Light
What sections should I know before attempting to learn this section?
—> Calculating the Wavelength and Frequency of Light
How do you calculate the energy of light?
We can also link the frequency calculations to the energy of a wave of light. When the frequency of a wave is HIGH the energy is LARGE. If the frequency is LOW the energy is SMALL. This relationship is also in the equation below.
Energy = Plank’s constant * (frequency)
E = h (f)
In the equation E represents energy and has the units of Joules (J), f stands for frequency and has the units of Hz, and h represents what they call Plank’s constant. Plank’s constant is 6.626 *10-34 m2kg/s.
Notice you can link together the equation for the energy of a wave and the equation for the speed of a wave because they both contain frequency.
VIDEO Energy of Light Calculation Demonstrated Example 1: If the frequency of light is 7.5 * 1021 Hz, how much energy does one this wave contain?
Step 1:
What information are we given?
Answer:
frequency = f = 7.5 * 1021 Hz
Plank’s constant = h = 6.626 * 10-34 m2kg/s (this is a constant that you should always have even if it does not state it in the problem)
Step 2:
What is the problem asking for?
Answer: Energy = E
Step 3:
What is the formula the question involves?
Answer: E = h (f)
Step 4:
How do we fill in the numbers for the formula?
Answer: E = 6.626 * 10-34 m2kg/s (7.5 * 1021 Hz)
Step 5:
COMPLETE ANSWER: about 5.0 * 10-12 J
VIDEO Energy of Light Calculation Demonstrated Example 2: If the wavelength of light is 2.8 * 104 m, how much energy does one of these waves contain?
Step 1:
What information are we given?
Answer:
wavelength = λ = 2.8 * 104 m
speed of light = c = 3.0 * 108 m/s (this is a constant that you should always have even if it does not state it in the problem)
Plank’s constant = h = 6.626 * 10-34 m2kg/s (this is a constant that you should always have even if it does not state it in the problem)
Step 2:
What is the problem asking for?
Answer: Energy = E
Step 3:
What is the first formula the question involves?
Answer: λ (f) = c
Step 4:
How do we fill in the numbers for the formula?
Answer: 2.8 * 104 m(f) = 3.0 * 108 m/s
Step 5:
How do we rearrange the equation to solve for frequency (f)?
Answer: Divide both sides by 2.8 * 104 m
| 2.8 * 104 m(f) = | 3.0 * 108 m/s |
| 2.8 * 104 m | 2.8 * 104 m |
Step 6:
Cross out like terms
| 2.8 * 104 m(f) = | 3.0 * 108 m/s |
| 2.8 * 104 m | 2.8 * 104 m |
Step 7:
Simplify
| f = | 3.0 * 108 m/s |
| 2.8 * 104 m |
Step 8:
What is the answer for the frequency (f)?
f = 10714 Hz or about 1.07 * 104 Hz
Step 9:
What is the second formula the question involves?
E = h (f)
Step 10:
How do we fill in the numbers for the formula?
E = 6.626 * 10-34 m2kg/s (1.07 * 104 Hz)
Step 11:
COMPLETE ANSWER: about 7.1 * 10-30 J
PRACTICE PROBLEMS: Solve for the unknown wavelength, frequency, or energy. Don’t forget you that you have your constants of speed of light = c = 3.0 * 108 m/s and Plank’s constant = h = 6.626 * 10-34 m2kg/s.
If the frequency of light is 3.5 * 106 Hz then what is the energy of the wave?
Answer: 2.3 * 10-27 J
If the energy of a light wave is 2.9 * 10-9 J, what is the frequency?
Answer: 4.4 * 1024 Hz
If the wavelength of a light wave is 1.4 * 10-8 m, what is the energy?
Answer: 1.4 * 10-17 J
If the energy of light is 8.7 * 10-11 J, what is the wavelength of the light?
Answer: 2.3 * 10-15 m
Chem – Calculating the Wavelength and Frequency of Light
What sections should I know before attempting to learn this section?
How do you calculate the wavelength or frequency of light?
In addition to the wavelength and frequency being related to the color of light, they are also related to each other. If you have a LONG wavelength then the frequency is LOW and if you have a SHORT wavelength then the frequency is HIGH. This relationship is also demonstrated in the equation below.
Wavelength * (Frequency) = speed
λ (f) = speed
The equation above is the general way to calculate the speed of a wave. However, the equation below is usually the one you see in a chemistry class. It is more specific to calculating the frequency and wavelength of the speed of light but all the concepts behind it are the same. Wavelength (λ) is measured in the units of meters (m). Frequency (f) is measured in the units of Hertz (Hz). The speed of light (c) is a constant number and its value is 3.0 * 108 m/s.
Wavelength * (Frequency) = speed of light
λ (f) = c
VIDEO Speed of light, Frequency, and Wavelength Calculation Demonstrated Example 1: If the frequency of the light is 240 Hz what is the wavelength?
Step 1:
What information are we given?
Answer:
frequency = f = 240 Hz
speed of light = c = 3.0 * 108 m/s (this is a constant that you should always have even if it does not state it in the problem)
Step 2:
What is the problem asking for?
Answer: wavelength = λ
Step 3:
What formula does this question involve?
Answer: λ (f) = c
Step 4:
How do we fill in the numbers for the formula?
Answer: λ (240 Hz) = 3.0 * 108 m/s
Step 5:
How do we rearrange the equation to solve for wavelength (λ)?
Answer: Divide both sides by 240 Hz
| λ (240 Hz) = | 3.0 * 108 m/s |
| 240 Hz | 240 Hz |
Step 6:
Cross out like terms
| λ (240 Hz) = | 3.0 * 108 m/s |
| 240 Hz | 240 Hz |
Step 7:
Simplify
| λ = | 3.0 * 108 m/s |
| 240 Hz |
Step 8:
COMPLETE ANSWER: 1.25 * 106 m
VIDEO Speed of Light, Frequency, and Wavelength Calculation Demonstrated Example 2: If the wavelength of light is 3.7 * 10-7 m what is the frequency?
Step 1:
What information are we given?
Answer:
wavelength = λ = 3.7 * 10-7 m
speed of light = c = 3.0 * 108 m/s (this is a constant that you should always have even if it does not state it in the problem)
Step 2:
What is the problem asking for?
Answer: wavelength = f
Step 3:
What formula does this question involve?
Answer: λ (f) = c
Step 4:
How do we fill in the numbers for the formula?
Answer:
| λ (3.7 * 10-7 m) = | 3.0 * 108 m/s |
| 1 |
Step 5:
How do we rearrange the equation to solve for frequency (f)?
Answer: Divide both sides by 3.7 * 10-7 m
| (3.7 * 10-7 m) f = | 3.0 * 108 m/s |
| 3.7 * 10-7 m | 3.7 * 10-7 m |
Step 6:
Cross out like terms
| (3.7 * 10-7 m) f = | 3.0 * 108 m/s |
| 3.7 * 10-7 m | 3.7 * 10-7 m |
Step 7:
Simplify
| f = | 3.0 * 108 m/s |
| 3.7 * 10-7 m |
Step 8:
COMPLETE ANSWER: 8.1 * 1014 Hz
PRACTICE PROBLEMS: Solve for the unknown wavelength or frequency. Don’t forget that you have the speed of light constant = c = 3.0 * 108 m/s.
If the wavelength of a light wave is 14.6m, what is the frequency?
Answer: 2.0 * 107 Hz
If the frequency of a light wave is 0.89 Hz, what is the wavelength?
Answer: 3.4* 108 m
If the wavelength of a light wave is 6.7 * 10-5 m, what is the frequency?
Answer: 4.5 * 1012 Hz
If the frequency of a light wave is 5.3 * 1012 Hz, what is the wavelength?
Answer: 5.7 * 10-5 m
Chem – Light and Electron Interactions
So what does the interaction of light and electrons look like?
Like light, electrons are also tiny particles. In addition, they move like light both very quickly and tend to follow wave patterns. What this means is that the particle of light, the photon, and the electron particle can interact. When these two particles (electrons and photons) interact, it turns out to be something like a pool (billiards) game. The photons can knock around the electrons and cause the electrons to move to different positions within an atom.
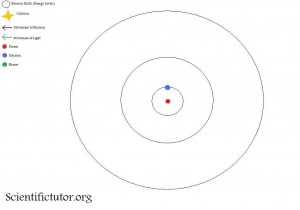
If we look at an atom as a Bohr model like the picture below, it allows us to gain a pretty easy understanding of the interactions between electrons and light. We will use the element hydrogen as our model atom. The first picture is what happens before the light particle (photon) hits the electrons. Notice how the electron starts on the closest electron shell (energy level) to the nucleus. The closest available shell (energy level) to the nucleus is where you will find the electron assuming no energy is put into it.
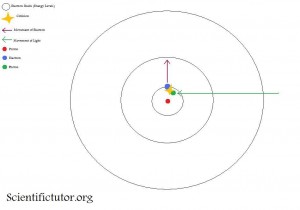
The second picture is what happens after the light particle (photon) hits the electron. See how the electron is moving from the first electron shell to the second electron shell. That was because the photon hit the electron and knocked or pushed it up one electron shell (energy level).
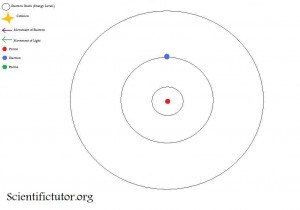
In the third picture, the electron has moved to the second shell.
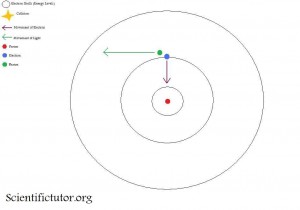
An instant (very very small) amount of time after the third, picture happens the fourth picture happens. The fourth picture is an electron falling back down to the closest shell to the nucleus while releasing a photon. It basically had this photon in storage and when it falls it releases it. Therefore, the light will travel away from the electron at this point.
In the fifth and last picture the electron in the hydrogen atom has returned to its original shell (energy level), closest to the nucleus.
This concept of electrons moving when they are struck by something else is actually the same basic concept that a soccer player uses to juggle a ball. Just like the soccer ball is being pulled to the Earth by gravity, the electron is being pull close to the nucleus because the electron (negatively charged) has an attraction to the protons (positively charged) in the nucleus. If you want to force the soccer ball away from the Earth, you have to knock (kick) your foot into the soccer ball. If you want to force the electron away from the nucleus, you have to knock the electron with a photon. When you knock the soccer ball, it flies away from the Earth and comes to some level for a split second and then falls back down. When you knock the electron, it flies away from the nucleus comes to a certain level for split second and then falls back down. You can even describe the energy that the electron stores as it rises and falls because light is a form of energy. The electron absorbs or releases that light energy as it changes position. Likewise, the soccer ball stores the energy in terms of sound. When you kick the soccer ball the collision of your foot and the ball make a sound. When the soccer ball falls back down it releases that sound once it hits something.
In fact, there is only one small difference between what happens with a soccer ball versus what happens with an electron. The electron follows a quantum (or is quantized) where as the soccer ball is not. Quantized means that the electron can only be at the first shell or the second shell or the third shell and so on. The electron cannot ever occupy the space in between the shells. So, if we run a video of the pictures in the example I gave you this is what it would look like. You see the electron at the first shell. You see the electron disappear. You see the electron reappear at the second shell. You see the electron disappear. You see the electron reappear at the first shell. It is kind of strange to think about but that is how it works. On the other hand, the soccer ball can move and occupy any space in between its starting point and its’ highest point after it is kicked by a person.
VIDEO Here is a video demonstration of the electron versus the football (soccer ball).
During the electron’s travel between different energy levels (shells), you can see different wavelengths of light coming off of the atom. The WAVELENGTH of light that the electron gives off when it falls back from a higher energy level to a lower energy level depends on the DISTANCE traveled by that electron. The GREATER the distance the HIGHER the energy, the HIGHER the frequency, and therefore the SHORTER the wavelength. For example, in most atoms, if an electron jumps only one energy level (shell) you will see a long wavelength (red side of the spectrum). However, if an electron jumps 4 or more energy levels then you will see a very short wavelength (purple side of the spectrum).
Chem – Light
What is light?
The technical name for light is electromagnetic radiation. Light is made up of tiny particles (spheres) called photons and is a form of energy. The sensation you get of feeling warmth on your skin when you walk outside on a sunny day is actually billions and billions of photon particles smashing into your skin and the reaction of your nerves sensing them. So, whenever there is light around, you are being bombarded by countless numbers of tiny particles. It is as if a thousand or more people all threw a ball at you at once and all those balls hit you. This is the easy part about light to understand. However, light also behaves in a second way. Unlike a ball or bullet, the photon of light does not travel in a straight line. The photon (particle) of light travels in a wave. That means it travels up and down slightly as it travels in the forward direction. This is kind of like a stone skipping across water except a stone will eventually sink, whereas the photon never stops the up and down motion. These two components, or behaviors of light,are what scientists call the wave particle duality. That is light acts both like a wave and a particle. A picture of the light wave is shown below. In science, anything that is called a wave or has wave properties travels in this way.
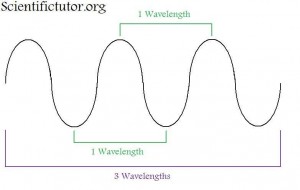
What is the difference between different colors of light?
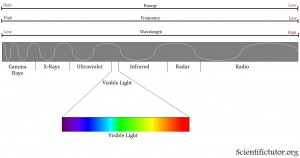
The most important feature of the wave is what they call the wavelength. A wavelength is the measurement of how long the wave is from crest to crest (highest point to the next highest point). You can also make the wavelength measurement at any repeating set of points like trough to trough (lowest point to lowest point). The difference in color of light that we see is the difference in how long or short those wavelengths are. If we look at a representation of the different colors that we see (the visible spectrum), we observe that the colors run from red to purple (violet) like in the electromagnetic spectrum picture below. Thereare also types of electromagnetic radiation that we, as humans, cannot see. Past the red side of the spectrum this includes things like radio waves and infrared. Past the purple side of the spectrum this includes things like ultraviolet and x-rays.
What is the relationship between the wavelength and the color of light?
Notice in the picture above that the red side of the spectrum has longer wavelengths than the purple side. Therefore, we can use color of light that we can see as a relative indicator of wavelength. Toward the RED side of the electromagnetic spectrum the WAVELENGTH IS LONGER. Toward the PURPLE side of the electromagnetic spectrum the WAVELENGTH IS SHORTER.
What is the frequency?
VIDEO Explanation of frequency.
Frequency is the other way to measure waves. In common terms it is how many times the wave goes up and down. In scientific terms, frequency is how many waves can you observe passing in a particular time period. The units of frequency are 1 / seconds or Hertz (Hz). A good way to think about frequency is to imagine you are standing at the edge of a body of water like a lake or ocean. Water waves behave the same laws as light waves so we can use water waves to test measurements and theories. Let’s bring a stopwatch and count how many waves reach our feet in one minute, then we can say something about the frequency of those waves. If a lot of waves hit your feet in one minute, like 10 waves, then the frequency of that particular set of waves is high. If only a handful of waves hit your feet in one minute, like 3 waves, then the frequency of that particular set of waves is low.
What is the relationship between frequency and the color of light?
We can look back to the electromagnetic spectrum and also see the relationship between frequency and color of light. Toward the RED side of the electromagnetic spectrum the FREQUENCY IS LOW. Toward the PURPLE side of the electromagnetic spectrum the FREQUENCY IS HIGH.
PRACTICE PROBLEMS: Given information about the wavelength or frequency, state whether the color of the light is more toward the red side of the spectrum or the purple side of the spectrum.
| Wave has a short wavelength | Purple side |
| Wave has a low frequency | Red side |
Chem – College: Rydberg Equation
How do we calculate the energy required to move an electron between energy levels?
The answer is the Rydberg equation. Note this Rydberg equation can only be used for hydrogen energy levels or shells. It is shown below. The equations are written two different ways. Both of the equations are the same but the second one is organized better for future demonstrated examples.
Δ E = RH((1/nI2) – (1/nf2))
| Δ E = | 1 – | 1 |
| RH | nI2 | nf2 |
The Δ E stands for the change in energy with the units of Joules (J). The RH is the Rydberg constant. It is simply the number 2.178 * 10-18 J that only applies to hydrogen and allows you to put the equation together. nI stands for the electron shell (or energy level) initial. It will be an integer like 1,2,3… The nf stands for the electron shell (or energy level) final and will also be an integer like 1,2,3…
The Rydberg equation also connects to other equations in this lesson. The most obvious one is E = h (f) in the section calculating the energy of light. Just treat Δ E and E as the same thing. The only difference between them is that Δ E can be a positive or negative number where as E has to be a positive number.
VIDEO Rydberg Equation Demonstrated Example 1: If the electron of a hydrogen atom goes from the second electron shell to the first electron shell, what is the maximum amount of energy that can be released as light? The Rydberg constant is 2.178 * 10-18 J.
Step 1:
What information are we given?
Answer:
nI = 2
nf = 1
RH = 2.178 * 10-18 J
Step 2:
What is the problem asking for?
Answer: Δ E = ?
Step 3:
What formula does the question involve?
Answer:
| Δ E = | 1 – | 1 |
| RH | nI2 | nf2 |
Step 4:
How do we fill in the numbers for the equation?
Answer:
| Δ E = | 1 – | 1 |
| 2.178 * 10-18 J | 22 | 12 |
Step 5:
How do we solve for the Δ E?
Answer: First simplify the exponents
| Δ E = | 1 – | 1 |
| 2.178 * 10-18 J | 4 | 1 |
Step 6:
Then put the fractions in decimals
| Δ E = | 0.25 – | 1 |
| 2.178 * 10-18 J |
Step 7:
Now subtract the right side
| Δ E = | – 0.75 | 1 |
| 2.178 * 10-18 J |
Step 8:
Finally multiply both sides by 2.178 * 10-18 J
| 2.178 * 10-18 J * Δ E = | – 0.75 * 2.178 * 10-18 J | 1 |
| 2.178 * 10-18 J |
Step 9:
Cross out like terms on left side
| 2.178 * 10-18 J * Δ E = | – 0.75 * 2.178 * 10-18 J | 1 |
| 2.178 * 10-18 J |
Step 10: simplify
| Δ E = | – 0.75 * 2.178 * 10-18 J | 1 |
| 1 |
Step 11: calculations
Answer: -0.75 * (2.178 * 10-18 J) = 1.6 * 10-18
| 1.6 * 10-18 = | – 0.75 * 2.178 * 10-18 J | 1 |
| 1 |
Step 12:
What is the final answer?
COMPLETE ANSWER: – 1.6 * 10-18 J
VIDEO Rydberg Equation Demonstrated Example 2: When a single hydrogen atom absorbs 2.09 * 10-18 J, and its electron starts from the first energy level, what energy level does the electron end at? The Rydberg constant is 2.178 * 10-18 J.
Step 1:
What information are we given?
Answer:
Δ E = 2.09 * 10-18 J
nI = 1
RH = 2.178 * 10-18 J
Step 2:
What is the problem asking for?
Answer: nf = ?
Step 3:
What formula does the question involve?
Answer:
| Δ E = | 1 – | 1 |
| RH | nI2 | nf2 |
Step 4:
How do we fill in the numbers for the equation?
Answer:
| 2.09 * 10-18 J = | 1 – | 1 |
| 2.178 * 10-18 J | 12 | nf2 |
Step 5:
How do we solve for nf?
Answer: First divide the left side.
| 0.96 = | 1 – | 1 |
| 12 | nf2 |
Step 6:
Then solve the exponent on the right side.
| 0.96 = | 1 – | 1 |
| 1 | nf2 |
Step 7:
Now solve the fraction on the right side.
| 0.96 = | 1 – | 1 |
| nf2 |
Step 8:
Minus 1 to both sides
| 0.96 – 1 = | 1 – 1 – | 1 |
| nf2 |
Step 9:
Cross out right side
| 0.96 – 1 = | 1 – 1 – | 1 |
| nf2 |
Step 10:
Simplify left side
| – 0.04 = | – | 1 |
| nf2 |
Step 11:
Eliminated the negatives from both sides
| 0.04 = | 1 | |
| nf2 |
Step 12:
Multiply both sides by nf2
| nf2 * 0.04 = | nf2 | 1 |
| nf2 |
Step 13:
Cross out like terms
| nf2 * 0.04 = | nf2 | 1 |
| nf2 |
Step 14:
Simplify
| nf2 * 0.04 = | 1 | |
| 1 |
Step 15:
Divide both sides by 0.04
| nf2 * 0.04 = | 1 | |
| 0.04 | 0.04 |
Step 16:
Cross out like terms
| nf2 * 0.04 = | 1 | |
| 0.04 | 0.04 |
Step 17:
Simplify
| nf2 = | 1 | |
| 0.04 |
Step 18:
Divide the right side ( 1 / 0.04 = 25 )
| nf2 = | 25 | |
| 1 |
Step 19:
Take the square root of both sides
| Sqrt nf2 = | Sqrt 25 | |
| 1 |
Step 20:
Cross out the square root and the square on the left side
| Sqrt nf2 = | Sqrt 25 | |
| 1 |
Step 21:
Simply
| nf = | Sqrt 25 | |
| 1 |
Step 22:
Take the square root of 25
| nf = | 5 | |
| 1 |
Step 23:
What is the final answer?
COMPLETE ANSWER: The 5th energy level
PRACTICE PROBLEMS: Solve the energy level (electron shell) or energy required to move electrons around in a hydrogen atom. Don’t forget to use the Rydberg constant RH of 2.178 * 10-18 J when needed.
If the electron of a hydrogen atom goes from the third electron shell to the first electron shell, what is the maximum amount of energy that can be released as light?
Answer: -1.94 * 10-18 J
If the electron of a hydrogen atom goes from the second electron shell to the fifth electron shell, what is the maximum amount of energy that can be absorbed as light?
Answer: 4.5 * 10-19 J
When a single hydrogen atom absorbs 2.04 * 10-18 J, and its electron starts from the first energy level, what energy level does the electron end at?
Answer: Fourth
When a single hydrogen atom releases -3.03 * 10-19 J, and its electron ends in the second energy level, what energy level does the electron start at?
Answer: Third
Chem – Electrons Moving Between Shells
How do electrons move between shells or energy levels?
VIDEO Explanation of electrons moving between shells.
With all this information about the position of the electrons compared to the nucleus in sections like electron shells and energy levels, we should also discuss how electrons can move from one shell or energy level to anther. In these next few sections I will use the words electron shells and energy levels to mean that same thing. Teachers can use either of those words to describe where electrons can move to. Make sure you understand both the sections on electron shells and energy levels before you go any further in this section.
So how do electrons move from one shell to the next? The explanation is actually quite simple. Let us discuss how an electron moves from a lower energy level to a higher energy level first. Electrons are negatively charged and move around the other edges of the atom, and protons are positively charged and are at the center of the atom (nucleus). That means that the negatively charged electron and the positively charged proton attract each other. So how do I move the electron away from the nucleus to a shell that is further away or to a higher energy level? Simple I expend energy or I put energy into the electron to do it. Pulling the electron away from the nucleus (center of an atom) requires the same kind of action just like moving an object off the floor against the force of gravity.
We can create a scenario that is easy to think about. I am going to use three objects or areas to describe it to you. Lets put a soccer ball on the ground. The ground represents the nucleus and the soccer ball represents the electron and the air above the ball represents higher energy levels. If I want to move the ball away from the ground and higher what do I do? I pick the ball up with my hands and move it away from the ground, but what is required for me to do that. I have to expend energy in my muscles and that energy gets stored in the ball. So moving the ball upward requires energy. The exact same requirements of energy are present when you move an electron away from the nucleus. In fact, the formula that allows us to calculate the force or energy between the electron and the proton (electromagnetic) is extremely similar to the formula that allows you to calculate the force or energy between the ball and the ground (gravity). Therefore, my analogy of the ball to the electron not only works well in thought, but also has mathematical proof behind it.
What if we now want to determine what happens when the electron moves from an outer shell or energy level to a lower shell or energy level? Again simple. We run the soccer ball analogy in reverse. If you are holding the soccer ball up high in your hands and you want it to move toward the floor what do you do? You drop it. What happens when you drop it? The ball moves toward the floor until something stops it and when the ball stops it lets out a sound. Sound is just a form or energy so all the ball is doing is letting go of some of its energy that was put into it when it became raised up. What does that mean the electron does? When the electron moves from a higher energy level to a lower energy level it must therefore give away some of its energy. However, the electron does not give away energy in the form of sound but in the form of light.
Chem – College: Quantum Numbers (Part 6)
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
—> Electron Configuration Diagram Part 5
—> Quantum Numbers Part 3 (mL )
—> Quantum Numbers Part 4 (ms )
—> Quantum Numbers Part 5 (All Together)
What are the possible quantum numbers?
The other common type of problem with quantum numbers is, what are the possible quantum numbers given in each of the quantum categories? For these questions I usually turn to a formula because it is easier to understand that way. You can also think about it conceptually if you want and I will explain this way as I go. In these problems they usually propose or give you one quantum category, like the energy level (n), and then ask you for the possibilities of another quantum category like the orbital types (L). The formulas I use to help me out are below.
n = number
L = n – 1 (and all integers to zero)
mL = L + the negative integers of L
ms = +1/2 or -1/2
How do you use these formulas? Pick a number for your energy level. For an example I am going to pick 3. (You can take a look at this periodic table if you don’t remember where your energy levels are) Take your energy level and minus 1 from it to get your possible L.
3 – 1 = 2….So L can equal 2, 1, 0. That means you can have an electron in the d, p, or s orbitals.
Now take your L possibilities add the negative integers to get ml.
2, 1, 0, (-1, -2)….So your ml can equal 2, 1, 0, -1, -2. That means in your d type orbitals you can have up to 5 orbitals labeled 2, 1, 0, -1, or -2. (Take a look at this periodic table if you don’t remember where your orbitals are.)
The last one, as always, is the easiest. No matter what the other quantum numbers are ms can only be either +1/2 or -1/2.
VIDEO Quantum Number Demonstrated Example 3: What are the possible quantum numbers if your n = 2?
Step 1:
If your n = 2 then what are your possible L?
n – 1 = L ….. 2 – 1 = 1
Answer: L = 1 or 0
Step 2:
If your L = 1 or 0 what are your possible mL?
Answer: mL = 1 or 0 or -1
Step 3:
What are your possible ms?
Answer: +1/2 or -1/2
COMPLETE ANSWER: L = 1,0, mL = 1,0,-1, ms = +1/2, -1/2
VIDEO Quantum Number Demonstrated Example 4: What are the possible quantum numbers if your mL = -3
Step 1:
If your ml = -3 what are your possible L?
We are dealing with the f orbitals here so the L for the f orbitals is 3
Answer: L = 3 or higher
Step 2:
If your L = 3 what are your possible n?
The first energy level that contains f orbitals is the 4th but energy levels beyond the 4th also have f orbitals.
Answer: n = 4 or higher.
Step 3:
What are your possible ms?
Answer: +1/2 or -1/2
COMPLETE ANSWER: n = 4 or higher, L = 3 or higher, ms = +1/2, -1/2
Notice, depending on which quantum number was picked for the question it can severely limit or open up the possibilities of the other quantum numbers.
PRACTICE PROBLEMS: What are the possible quantum numbers for the following problems? Remember to have your regular periodic table handy. Also if you need it have the quantum periodic table and the energy level periodic table ready.
| L = 2 | n = 3,4,5… mL = 2,1,0,-1,-2 ms = +1/2, -1/2 |
| n = 4 | L = 3,2,1,0 mL = 3,2,1,0,-1,-2,-3 ms = +1/2, -1/2 |
| mL= 1 | n = 2,3,4… mL = 1,0,-1 ms = +1/2, -1/2 |
Chem – Quantum Numbers (Part 5)
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
—> Electron Configuration Diagram Part 5
—> Quantum Numbers Part 3 (mL )
—> Quantum Numbers Part 4 (ms )
How do we use the 4 different types of quantum numbers together in one answer?
Now that we know what each quantum number means we also want to be able to put all the different quantum numbers together to answer one question. You may have noticed that I have used the exact same elements as my example problems for all the different quantum categories. That is because for most questions asked about quantum numbers they usually go one of two ways. Tests tend to ask either what are the all the quantum numbers for the last electron in a certain element? Or they tend to ask what are the possible quantum numbers given in each of the quantum categories? We will go through each type of question below.
The first question, what are all the quantum numbers for the last electron in a certain element is answered by analyzing the energy level (n), orbital type (L), specific orbital (mL), and spin of the electron (ms). This is very similar to the practice problems we have had so far it is just combining them all into one.
Remember to use the periodic table links like your regular periodic table handy. Also if you need it have the quantum periodic table and the energy level periodic table ready.
VIDEO Quantum Number Demonstrated Example 1: What are the quantum numbers for the last electron of phosphorus? Remember to use these periodic table links if you need them.
Step 1:
What energy level is phosphorus in?
Answer: The third energy level…..so n = 3
Step 2:
What orbital type is phosphorus in?
Answer: The p orbitals…..so L = 1
Step 3:
What specific orbital is phosphorus in?
Answer: The second p orbital…..so mL = 1
Step 4:
What is the spin of the last electron in phosphorus?
Answer: ms = -1/2
Step 5:
What are the quantum numbers for the last electron of phosphorus?
COMPLETE ANSWER: n = 3, L = 1, mL = 1, ms = -1/2
VIDEO Quantum Number Demonstrated Example 2: What are the quantum numbers for the last electron of yttrium? Remember to use these periodic table links if you need them.
Step 1:
What energy level is yttrium in?
Answer: The fourth energy level…..so n = 4
Step 2:
What orbital type is yttrium in?
Answer: The d orbitals…..so L = 2
Step 3:
What specific orbital is yttrium in?
Answer: The first d orbital…..so mL = -2
Step 4:
What is the spin of the last electron in phosphorus?
Answer: ms = -1/2
Step 5:
What are the quantum numbers for the last electron of phosphorus?
COMPLETE ANSWER: n = 4, L = 2, mL = -2, ms = -1/2
Examples: What are the quantum numbers for the last electron of each element below? Remember to have your regular periodic table handy. Also if you need it have the quantum periodic table and the energy level periodic table ready.
| Ca | n = 4, L = 0, mL = 0, ms = +1/2 |
| Mo | n = 4, L = 2, mL = 1, ms = -1/2 |
| In | n = 5, L = 1, mL = -1, ms = -1/2 |
PRACTICE PROBLEMS: What are the quantum numbers for the last electron of each element below? Remember to have your regular periodic table handy. Also if you need it have the quantum periodic table and the energy level periodic table ready.
| Si | n = 3, L = 1, mL = -1, ms = -1/2 |
| O | n = 2, L = 1, mL = 0, ms = +1/2 |
| Mn | n = 3, L = 2, mL = 2, ms = -1/2 |
| Rb | n = 5, L = 0, mL = 0, ms = -1/2 |
| Th | n = 5, L = 3, mL = -2, ms = -1/2 |
| Kr | n = 4, L = 1, mL = 1, ms=+1/2 |
Chem – Quantum Numbers (Part 4)
What sections should I know before attempting to learn this section?
—> Electron Configuration Diagram Part 5
What does the ms stand for in the quantum numbers?
The last quantum category is ms (It is pronounced “M sub S”) represents what they call the spin. It is the easiest to figure out and the easiest to understand. Just like in the electron diagrams we used an up and a down arrow to represent the electrons traveling different directions within the same orbital. Here instead we use +1/2 and -1/2 to represent the up and down respectively. You can honestly choose these things at random so long as no two electrons in a row (within the same orbital) have the same ms. You can put them in a specific order on the periodic table but it does not matter that much. I usually put +1/2 first. This is one example of a periodic table where the electron spin is illustrated.
Examples: Give the ms for the last electron in the following elements. (These do not have definite answers but I give the answer I do because of the way I have explained them to you.)
| Be | ms = -1/2 |
| Pd | ms = -1/2 |
| Al | ms = +1/2 |
PRACTICE PROBLEMS: Give the ms for the last electron in the following elements. (These do not have definite answers but I give the answer I do because of the way I have explained them to you.)
| Bi | ms = +1/2 |
| F | ms = -1/2 |
| Ni | ms = -1/2 |
| Li | ms = +1/2 |
Chem – Quantum Numbers (Part 3)
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
What does the mL stand for in the quantum numbers?
The next quantum number category mL (it is pronounced “M sub L”) stands for the specific orbital within the orbital types. This relates back to how many orbitals each orbital type has. The s orbitals have one orbital each. The p orbitals have 3 orbitals each. The d orbitals have 5 orbitals each. The f orbitals have 7 orbitals each. The mL depends on the (L). The table below will show you best how ml is dependent on (L).
| If (L) is | Then mL can be… | Orbital types | # of electrons | # of orbitals |
| L = 0 | 0 | s | 2 | 1 |
| L = 1 | -1, 0, 1 | p | 6 | 3 |
| L = 2 | -2, -1, 0, 1, 2 | d | 10 | 5 |
| L = 3 | -3, -2, -1, 0, 1, 2, 3 | f | 14 | 7 |
Notice in the table above the two columns I highlight in red are related. On the bottom row there are 7 integers running from negative 3 to positive 3. Each one of those integers represents an individual orbital among that orbital type. Again you can reference the quantum periodic table to show you better where they are and how they are arranged. Keep in mind that different teachers like to display them in slightly different ways. The way I demonstrate on the periodic table seems to be the easiest to understand but it is not the only way to understand this concept.
Examples: Give the mL for the last electron in the following elements.
| Be | mL = 0 |
| Pd | mL = 1 |
| Al | mL =-1 |
PRACTICE PROBLEMS: Give the mL for the last electron in the following elements.
| K | mL = 0 |
| S | mL = 0 |
| Ge | mL = -1 |
| Ti | mL = -2 |
| Au | mL = 2 |
| Lu | mL = 3 |
Chem – Quantum Numbers (Part 2)
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
What does the L stand for in quantum numbers?
The next quantum number category L stands for the type of orbitals. In electron configurations the types of orbitals were the s, p, d, f orbitals that were discussed in the orbitals section of this lesson. In the quantum numbers if L = 0 represents the s orbitals. If L = 1 represents the p orbitals. If L = 2 represents the d orbitals. If L = 3 represents the f orbitals. Another way to represent that is the table below.
| L = | Orbital types |
| 0 —> | s |
| 1 —> | p |
| 2 —> | d |
| 3 —> | f |
You can also see how this is represented on the quantum periodic table with this link.
Examples: Give the L (orbital type) of the last electron in each element.
| Be | L = 0 |
| Pd | L = 2 |
| Al | L = 1 |
PRACTICE PROBLEMS: Give the L (orbital type) of the last electron in each element.
| P | L = 1 |
| Co | L = 2 |
| Sn | L = 1 |
| Ba | L = 0 |
| Th | L = 3 |
| W | L = 2 |
Chem – Quantum Numbers Introduction (Part 1)
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
What are quantum numbers?
VIDEO Explanation of quantum numbers.
Quantum numbers and electron configurations are talking about the same thing. Each of them represent where the electrons are around the atom. However, the quantum numbers do it in a different way than the electron configurations do. The first difference is the quantum numbers only talk about one electron at a time. The quantum numbers also divide into 4 different categories represented by letters. Those categories are n, L, mL, ms.
The quantum category n stands for the energy level. This is the same energy level that was discussed in the earlier energy level section of this lesson. This periodic table link can help you recognize the energy levels on the periodic table.
Examples: Give the n (energy level) of the last electron in each element.
| Be | n = 2 |
| Pd | n = 4 |
| Al | n = 3 |
PRACTICE PROBLEMS: Give the n (energy level) of the last electron in each element.
| He | n = 1 |
| Fe | n = 3 |
| Br | n = 4 |
| Na | n = 3 |
| U | n = 5 |
| Hg | n = 5 |
Chem – Electron Configuration Diagrams
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
—> Abbreviated Electron Configuration Part 4A
What are electron configuration diagrams?
VIDEO Explanation of electron configuration diagrams.
Electron configuration diagrams are how to represent the electron configurations in a picture form. Mainly what you need to know is how the picture below this paragraph is created. I will explain it in words as you are simultaneously looking at the picture below. You might also want to bring up the energy level periodic table. The electron configuration picture is displayed by putting the orbitals closest to the nucleus (of the lowest energy) further toward the bottom of the picture. As the orbitals get further from the nucleus (higher in energy) they are displayed higher and higher in the picture. For example, the 3p orbitals are higher on an electron configuration diagram then the 2s orbital. The orbital types (s, p, d, f…) are also organized in a specific way. All the s orbitals are lined up furthest toward the left side of the picture. The s orbitals are also stacked one on top of the next. The p orbitals are slightly to the right of the s orbitals and slightly above the s orbitals in the same energy levels. For example, the 2p orbital is to the right of and slightly higher than 2s orbitals. However, the p orbitals like the s orbitals, are stacked one on top of another.
What is each box representing in the picture above? Each box represents one orbital. Each orbital can only hold 2 electrons maximum. DO NOT CONFUSE THIS WITH THE ORBITAL TYPES. If you take the maximum number of electrons that an orbital type can hold and divide it by 2, you get the amount of orbitals in that orbital type. For example, the d orbitals can hold 10 electrons. So, 10 divided by 2 equals 5. That is why the picture above has 5 boxes representing the 3d orbitals. If we are only able to put 2 electrons in each of the d orbitals, then we can put a total of 10 electrons in all the 3d orbitals. In the electron configuration diagram above, the electrons are represented by the up and down arrows. It does not matter if it is an up or down arrow. Both of them represent an electron. The only difference between the up and down arrow is the direction the electrons are traveling. The up versus down arrows are saying that the electrons are traveling in opposite directions relative to one another. I describe this movement of electrons by comparing it to cars on a racetrack. If I were representing cars on a racetrack by arrows, I would make them go in all the same direction (all up), because all cars go in the same direction during the race. However, what if I took half the cars in my race and made them go in the other direction? Besides a lot of potential car accidents happening, you could then show the cars running the races as half of them being up arrows and half of them being down arrows. This is just like electrons which race toward each other within the same track (orbital). Although the electrons do not collide with each other because they are both negatively charged, the cars around a racetrack versus electrons in an orbital is a very close analogy to what is actually happening in an atom or orbital.
To determine which orbitals are closest to the nucleus (lowest in energy), you have to break down your determination into two categories. The first is what energy level is that orbital on. The second is what type of orbital is it. Orbitals of a lower energy level will be closer to the nucleus. For example, the 2p orbitals will be closer to the nucleus than the 4s orbitals because the 2p orbitals is on the second energy level and the 4s are on the fourth energy level. Also, there is an order of orbitals within the same energy level. The s orbitals will be closer to the nucleus than the p orbital within the same energy level. The p orbitals will be closer to the nucleus than the d orbital within the same energy level. The d orbitals will be closer to the nucleus than the f orbital within the same energy level. For example, the 3p orbitals are closer to the nucleus than the 3d orbitals.
Examples: Which orbitals are CLOSEST (HAVE THE LOWEST ENERGY) to the nucleus?
| 3s or 1s | 1s |
| 4f or 2p | 2p |
| 5d or 5f | 5d |
PRACTICE PROBLEMS: Which orbitals are CLOSEST (HAVE THE LOWEST ENERGY) to the nucleus?
| 5f or 4f | 4f |
| 7p or 6s | 6s |
| 2s or 2p | 2s |
| 4d or 4p | 4p |
| 3d or 4p | 3d |
| 6f or 6s | 6s |
Now that we have a general understanding of the number of orbitals, orbital types, and the relationship of orbitals to the nucleus we should be able to start constructing electron configuration diagrams. How do we do that? We should first open up a periodic table with the energy levels and orbital types clearly displayed. Here is the same periodic table viewed in a different way. Just like the electron configures before, we want to start any electron configuration by starting at the top of the periodic table in the 1s orbitals or the spot where hydrogen is. Since it is very hard to explain electron diagrams in text, there are video examples below.
VIDEO Example 1 Nitrogen: How to draw an electron diagram.
VIDEO Example 2 Sulfur: How to draw an electron diagram.
VIDEO Example 3 Iron: How to draw an electron diagram.
PRACTICE PROBLEMS: Draw the electron diagram of the following elements or charged atoms. Use this periodic table if possible. If that does not work for you try the orbital periodic table. It is best to draw them from memory but you can refer to a template electron diagram if you wish.
| B | Answer 1 link |
| F | Answer 2 link |
| Ca | Answer 3 link |
| Rh2+ | Answer 4 link |
| Se2- | Answer 5 link |
| Ru | Answer 6 link |
Chem – College: Exceptions to the Electron Configurations
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
—> Abbreviated Electron Configuration Part 4A
Why can electrons configurations in the D orbitals change?
Some electron configurations tend to fill electrons in an odd way. These are the electron configurations that are exceptions to the rule. They mainly tend to focus on the s-orbitals and d-orbitals since these two orbitals are about the same distance from the nucleus (they have about the same energy). These exceptions to the rules start when you enter the 4s and 3d region of the orbitals displayed on the periodic table orbitals link here. The 4s orbitals are slightly closer to the nucleus (slightly lower energy) than the 3d so that is why the 4s orbitals are displayed first on the periodic table. However, they do not always fill first. It depends on the exact number of electrons or electron arrangement that a given element has. The exceptions come from one over arching concept. The d-orbitals prefer to have either 1 electron in each orbital or 2 electrons in each orbital. These two arrangements of electrons in the d-orbitals are more stable and therefore tend to happen when it is possible.
Examples: Give the abbreviated (noble gas) electron configurations for the elements below. Use the orbital periodic table.
| V | [Ar] 3d5 |
| Cr | [Ar] 4s13d5 |
| Ni | [Ar] 3d10 |
| Cu | [Ar] 4s13d10 |
VIDEO Electron Configuration Exceptions Demonstrated Example 1: What is the abbreviated electron configuration for Mo? Use the orbital periodic table.
Step 1:
What is the nearest noble gas above Mo?
Answer: Kr
What should be written down so far: [Kr]
Step 2:
From Kr where do we continue the electron configuration?
Answer: into the 5s or 4d.
Step 3:
How many total electrons are in the 5s and 4d orbitals?
Answer: 6
Step 4:
Where do I distribute the electrons between the 5s and 4d orbitals?
Answer: first give 5 electrons to the 4d orbitals. Then give whatever is left over to the 5s orbitals. In this case the 5s orbitals will have 1 electron.
COMPLETE ANSWER: [Kr] 5s14d5
PRACTICE PROBLEMS: Give abbreviated (noble gas) electron configurations for the elements or charged atoms below. Use this periodic table if possible. If that does not work for you try the orbital periodic table.
| Ag | [Kr] 5s14d10 |
| Nb | [Kr] 4d5 |
| Pd | [Kr] 4d10 |
| Fe3+ | [Ar] 3d5 |
| Cd2+ | [Kr] 4d10 |
Chem – Abbreviated Electron Configurations
What sections should I know before attempting to learn this section?
—> Complete Electron Configuration Part 3
What are abbreviated electrons configurations?
Because some of the complete electron configurations can be so long, chemical scientists have come up with a way to shorten them. It involves using the closest noble gas above the element you are trying to give an electron configuration to.
Examples: Give the abbreviated (noble gas) electron configurations for the elements below. Use the orbital periodic table and the regular periodic table together.
| Element | Normal Configuration | Noble Gas Configuration |
| Li | 1s22s1 | [He] 2s1 |
| P | 1s22s22p63s23p3 | [Ne] 3s23p3 |
VIDEO Abbreviated Electron Configurations Demonstrated Example 1: What is the abbreviated electron configuration for Sr? Use the orbital periodic table and the regular periodic table together.
Step 1:
What is the nearest noble gas above Sr?
Answer: Kr
What should be written down so far: [Kr]
Step 2:
From Kr where do we continue the electron configuration?
Answer: at 5s
What should be written down so far: [Kr] 5s
Step 3:
How many boxes (electrons) on the periodic table until we get to Sr?
Answer: 2
COMPLETE ANSWER: [Kr] 5s2
PRACTICE PROBLEMS: Give abbreviated (noble gas) electron configurations for the elements below. Use this periodic table if possible. If that does not work for you try the orbital periodic table.
| S | [Ne] 3s23p4 |
| K | [Ar] 4s1 |
| Bi | [Xe] 6s24d145d106p3 |
| Fe | [Ar] 4s23d6 |
| Ne | [He] 2s22p6 |
| Rf | [Rn] 7s25f146d2 |
Chem – Complete Electron Configurations
What sections should I know before attempting to learn this section?
What are complete electrons configurations?
VIDEO Explanation of complete electron configurations.
Electron configurations are a map of the how the electrons fill up the different orbitals in order. The electron configurations break into 3 different symbols that each have their own meaning. One example is below:
3p5
The first notation, the number 3, stands for the energy level. The second notation, the letter P, stands for the orbital type. The third and final notation, the exponent or superscript 5, stands for how many electrons are currently filling up those orbitals. The purpose of the questions about the complete electron configurations is for you become familiar with which energy levels and orbitals are closest to the nucleus and thus fill up with electrons first. It is one way to represent where the electrons are in a single atom and can help you predict how bonds form between that atom and other atoms. The complete electron configurations can be related to the periodic table by showing the energy levels and orbitals like on this link. If this is still confusing, there is another way to visualize the electron configurations according to the energy level diagram that I showed earlier in the previous section of energy levels. I will explain how to solve complete electron configuration questions by focusing on how to relate them to the periodic table because I think that is a far easier and better method to learn.
Examples: Give the complete electron configurations for the following pure elements. Use the orbital periodic table.
| Li | 1s22s1 |
| P | 1s22s22p63s23p3 |
| Br | 1s22s22p63s23p64s23d104p5 |
| O2- | 1s22s22p6 |
VIDEO Complete Electron Configurations Demonstrated Example 1: What is the complete electron configuration of Ti? (Solve while allowing your finger to follow along on the periodic table orbitals) Write down each piece of information you learn in order, as you go. You may also need a regular periodic table for reference of where each element is.
Step 1:
Where do you start the problem?
Answer: open up the link to the periodic table orbitals and place your eye or finger at the first element (hydrogen).
Step 2:
What energy level are you at on the periodic table with that first element (hydrogen)?
Answer: 1
What should be written down so far: 1
Step 3:
What orbital type is the first element (hydrogen) in?
Answer: s
What should be written down so far: 1s
Step 4:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s2
Step 5:
Move to the next orbitals on the periodic table on the periodic table (the next row in this case). What element do you start at if you do?
Answer: Li
What should be written down so far: 1s2
Step 6:
What energy level is lithium at?
Answer: 2
What should be written down so far: 1s22
Step 7:
What orbital type is lithium at?
Answer: s
What should be written down so far: 1s22s
Step 8:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s22s2
Step 9:
Move to the next orbitals on the periodic table. What element do you start at if you do?
Answer: B
What should be written down so far: 1s22s2
Step 10:
What energy level is Boron at?
Answer: 2
What should be written down so far: 1s22s22
Step 11:
What orbital type is Boron at?
Answer: p
What should be written down so far: 1s22s22p
Step 12:
Count the boxes across the P orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 6
What should be written down so far: 1s22s22p6
Step 13:
Move to the next orbitals on the periodic table (the next row in this case). What element do you start at if you do?
Answer: Na
What should be written down so far: 1s22s22p6
Step 14:
What energy level is Sodium at?
Answer: 3
What should be written down so far: 1s22s22p63
Step 15:
What orbital type is Sodium at?
Answer: s
What should be written down so far: 1s22s22p63s
Step 16:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s22s22p63s2
Step 17:
Move to the next orbitals on the periodic table. What element do you start at if you do?
Answer: Al
What should be written down so far: 1s22s22p63s2
Step 18:
What energy level is Aluminum at?
Answer: 3
What should be written down so far: 1s22s22p63s23
Step 19:
What orbital type is Aluminum at?
Answer: p
What should be written down so far: 1s22s22p63s23p
Step 20:
Count the boxes across the P orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 6
What should be written down so far: 1s22s22p63s23p6
Step 21:
Move to the next orbitals on the periodic table (the next row in this case). What element do you start at if you do?
Answer: K
What should be written down so far: 1s22s22p63s23p6
Step 22:
What energy level is Potassium at?
Answer: 4
What should be written down so far: 1s22s22p63s23p64
Step 23:
What orbital type is Potassium at?
Answer: s
What should be written down so far: 1s22s22p63s23p64s
Step 24:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s22s22p63s23p64s2
Step 25:
Move to the next orbitals on the periodic table. What element do you start at if you do?
Answer: Sc
What should be written down so far: 1s22s22p63s23p64s2
Step 26:
What energy level is Scandium at?
Answer: 3
What should be written down so far: 1s22s22p63s23p64s23
Step 27:
What orbital type is Scandium at?
Answer: d
What should be written down so far: 1s22s22p63s23p64s23d
Step 28:
Count the boxes across the D orbitals until you reach TITANIUM. That should represent the number of electrons. How many boxes did you count?
Answer: 2
COMPLETE ANSWER: 1s22s22p63s23p64s23d2
It is also possible to determine the electron configurations of charged elements. What you have to take into account for charged elements is that they will have gained or lost electrons to look like the electron configuration of another element on the periodic table. Another way to phrase this is that the charged element is isoelectric with another element.
VIDEO Complete Electron Configurations Demonstrated Example 2: What is the complete electron configuration of Al3+? (Solve while allowing your finger to follow along on the periodic table orbitals) Write down each piece of information you learn in order, as you go. You may also need a regular periodic table for reference of where each element is.
Step 1:
Al3+ has electrons that look like what element on the periodic table?
Answer: Count back on the periodic table 3 spaces. It is Ne
Therefore we now look for the electron configuration of Ne.
Step 2:
What energy level are you at on the periodic table with that first element (hydrogen)?
Answer: 1
What should be written down so far: 1
Step 3:
What orbital type is the first element (hydrogen) in?
Answer: s
What should be written down so far: 1s
Step 4:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s2
Step 5:
Move to the next orbitals on the periodic table on the periodic table (the next row in this case). What element do you start at if you do?
Answer: Li
What should be written down so far: 1s2
Step 6:
What energy level is lithium at?
Answer: 2
What should be written down so far: 1s22
Step 7:
What orbital type is lithium at?
Answer: s
What should be written down so far: 1s22s
Step 8:
Count the boxes across the S orbitals until you reach the end. That should represent the number of electrons. How many boxes did you count?
Answer: 2
What should be written down so far: 1s22s2
Step 9:
Move to the next orbitals on the periodic table. What element do you start at if you do?
Answer: B
What should be written down so far: 1s22s2
Step 10:
What energy level is Boron at?
Answer: 2
What should be written down so far: 1s22s22
Step 11:
What orbital type is Boron at?
Answer: p
What should be written down so far: 1s22s22p
Step 12:
Count the boxes across the P orbitals until you reach NEON. That should represent the number of electrons. How many boxes did you count?
Answer: 6
COMPLETE ANSWER: Al3+ = 1s22s22p6
PRACTICE PROBLEMS: Give the complete electron configurations of the following elements or charged atoms below. Use this periodic table if possible. If that does not work for you try the orbital periodic table.
| Mg | 1s22s22p63s2 |
| As | 1s22s22p63s23p6s23d104p3 |
| Zn | 1s22s22p63s23p64s23d10 |
| Nd | 1s22s22p63s23p6s23d104p35s24d105p66s24f4 |
| Sn2+ | 1s22s22p63s23p6s23d104p35s24d10 |
| Rb | 1s22s22p63s23p6s23d104p35s1 |
| S2- | 1s22s22p63s23p6 |
Chem – Energy Levels
What sections should I know before attempting to learn this section?
What are energy levels?
Now that we have talked about the orbitals we can start to talk about how they are related to more complex subjects like energy levels. Energy levels refer to the distance of electrons from the nucleus. The further the electron is from nucleus the greater energy it has. Just like the higher you raise a book from the ground the greater energy it has. In both cases, if you allow the electron or the book to fall, the energy will be released. The electron with release the energy in the form of light and the book will release the energy in the form of the loud crashing sound. The energy levels are depicted on the periodic table and relate to the different orbitals discussed above. They ROUGHLY follow the periods of the periodic table but it depends on what type of orbital you are viewing. The best way to understand the energy levels is to compare the energy level diagram with the energy levels on the periodic table. I demonstrate this in the video below.
For the energy level diagram, the numbers in front of the different orbital types are the indications of the energy levels. You can see that the first energy level only has the S orbital. However, the second energy level has both the S and P orbitals. If we keep going, the third energy level has the S, P, and D orbitals. So, each time we move down an energy level we add a new orbital type. The picture I have above is not complete. Theoretically you could go on forever. However, I show it up to the 5th energy level because most students do not need to know beyond that. Also, at the 5th energy level it starts to get into theoretical orbitals like the G orbital that don’t appear on the periodic table. All you have to know about the theoretical orbitals is that they start in the fifth energy level at G and then go in alphabetical order (G, H, I…) as you continue down the energy level diagram. Questions about energy levels often revolve around how many electrons each of them can hold.
Examples: How many electrons can each of these energy levels hold?
| First energy level | 2 |
| Third energy level | 18 |
VIDEO Counting Electrons in Energy Levels Demonstrated Example 1: How many electrons are held in the first, second, and third energy levels total? Try this link to a periodic table with the energy levels on it as you go through this problem.
Step 1:
What type of orbitals are in the 1st energy level?
Answer: just S
Step 2:
How many electrons do the S orbitals hold?
Answer: 2
Step 3:
So how many electrons are in 1st energy level?
Answer: 2
Step 4:
What type of orbitals are in the 2nd energy level?
Answer: S and P
Step 5:
How many electrons do the P orbitals hold?
Answer: 6
Step 6:
So how many electrons are in the 2nd energy level?
Answer: S + P = Total …… 2 + 6 = 8
Step 7:
What type of orbitals are in the 3rd energy level?
Answer: S, P, and D
Step 8:
How many electrons do the D orbitals hold?
Answer: 10
Step 9:
So how many electrons in the 3rd energy level?
Answer: S + P + D = Total …… 2 + 6 + 10 = 18
Step 10:
How many electrons in the first 3 energy levels?
Answer: First + Second + Third = Total …… 2 + 8 + 18 = 28
COMPLETE ANSWER: 28 electrons
PRACTICE PROBLEMS: How many electrons can each of these energy levels hold? Try to use a regular periodic table when you answer these. If the regular periodic table is not enough try this link to a periodic table with the energy levels on it as you go through these problems.
| Second energy level | 8 |
| Fourth energy level | 32 |
Chem – Orbitals
What are orbitals?
VIDEO Explanation of orbitals.
If you look deeper into the paths that the electrons take around the nucleus of an atom you actually begin to see that some of the shapes are not circular or spherical like the Bohr model suggests. Therefore, the Bohr model we have been working with so far is not the complete story of how electrons travel around the nucleus. Because Bohr studied the hydrogen atom and because the most common paths that the electrons take in a hydrogen atom are spherical, it is understandable that he missed the other more oddly shaped electron paths. Although Bohr’s work was ground–breaking and brilliant, we now have a better picture of the electron paths around the atom. Instead of calling them shells we now refer to the paths that the electrons take as orbitals. The first type of orbital is the S orbital. S stands for spherical and these are the orbitals that Bohr discovered. However, the other orbitals tend to be dumbbell shaped. Those orbitals include the P, D, and F. The F orbitals also have some strange doughnut shaped orbitals. It is not very important to know the shapes of the different orbitals, but it is good to know that they have different shapes.
The two most important aspects of the orbitals are to be able to spot the orbitals on the periodic table and to know how many electrons are in each orbital set. Because of the organization of the periodic table, you can also find where the different orbitals are. The periodic table orbitals is a good link to view as you read this these next sentences. There is also an alternative way to look at the orbitals on the periodic table with this link (shifted). The first set of orbitals is the S orbitals. The S orbitals are the first two columns of the periodic table that start with Hydrogen and Lithium. Helium is also part of the S orbitals even though it is on the right side of the periodic table. This means that the last electron in hydrogen and helium travels around the atom in an S orbital shape. If we count through the rows of the periodic table we notice that each row contains only two elements in the S orbitals. That means the S orbitals can carry a maximum of 2 electrons.
The next orbitals are the P orbitals. The P orbitals start on the second row of the periodic table with boron and carbon. The P orbitals continue all the way to the element neon in the second row of the periodic table. If we count across that row from boron to neon we find that each P orbital set contains a maximum of 6 electrons. All elements below the columns that start with boron, carbon, nitrogen, oxygen, fluorine, and neon are considered to have their last electron in the P orbitals. D orbitals roughly follow the transition metals. On the fourth row of the periodic table the D orbitals start with scandium and end with zinc. If we count across from scandium to zinc, we discover that the D orbitals contain a maximum of 10 electrons. The D orbitals are all those columns between and including scandium and zinc. The final orbitals shown on the periodic table are the F orbitals. The F orbitals are the separated elements down at the bottom of the periodic table. The top row of the F orbitals begins with Lanthanum and ends with Ytterbium. The F orbitals are also the next row below cerium and lutetium. If we count across from cerium to lutetium, we learn that the F orbitals contain a maximum of 14 electrons.
You should have a basic understanding of how the first few orbitals fit together into a picture. VIDEO to show you how to draw the first few orbitals.
Examples: The last electron of each element is in what type of orbital? Use the periodic table orbitals link as you are analyzing these examples. If you need to, use the The periodic table orbitals.
| Element | Orbital Type |
| C | p |
| Sr | s |
| U | f |
| Ni | d |
| Sn | p |
PRACTICE PROBLEMS: The last electron of each element is in what type of orbital? Try using a regular periodic table to solve these. Try NOT to use the The periodic table orbitals.
| Element | Orbital Type |
| Fe | d |
| He | s |
| Al | p |
| Pu | f |
| Xe | p |
| K | s |
| Hg | d |
Chem – LESSON 5: Electron Orbitals and Light
What is this lesson about?
This lesson is mostly about how to visualize the specific paths or orbitals that electrons take around the atom and how some of those paths are more stable or less stable. It will also teach you how electrons can move in 3 dimensions instead of the 2 dimensions we have modeled in the previous section. How light and electrons interact is the last fascinating part of this lesson.
Why is it critical to understand?
A lot of the discoveries in this field were the foundations for particle physics. This information was used to help calculate things like the speed and mass of an electron. The visualization of electrons traveling in 3 dimensions also helps you explore more advanced concepts in lessons like Valence Electron Dot Structures (Lewis structures), coordinate covalent compounds and special interactions with transition metals.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to sections on Protons and Electrons, Ions, Equations, and Solving for an Unknown.
New Learning Sections:
—> Complete Electron Configuration Part 3
—> Abbreviated Electron Configuration Part 4A
—> College: Exceptions to the Electron Configuration Part 4B
—> Electron Configuration Diagram Part 5
—> Quantum Numbers Part 3 (mL )
—> Quantum Numbers Part 4 (ms )
—> Quantum Numbers Part 5 (All Together)
—> College: Quantum Numbers Part 6 (possible numbers)
—> Electrons Moving Between Shells
—> Light
—> Light and Electron Interactions
—> Calculating the Wavelength and Frequency of Light
—> Calculating the Energy of Light
Reference Pages:
—> Orbitals on the Periodic Table
—> Energy Levels on the Periodic Table
—> Template of Electron Configuration Diagram
—> Equations Involving Orbitals and Light
Worksheets:
—> Electron Orbitals Worksheet 1
—> Electron Orbitals Worksheet 1 WITH ANSWERS
Chem – College: Causes of Periodic Trends
What causes the periodic trends?
VIDEO Explanation for cause of Periodic Trends
To ask about why the periodic trends exist is to ask a very fundamental question about how the structure of the atom and the structure of how the universe itself is put together. To understand we start off with what is called Coulomb’s Law. Coulomb’s Law is an equation that describes one of the few forces that holds our universe together (similar to gravity). Coulomb’s Law can tell us about more than one possible scenario but in this case we are going to use it to talk about how the proton (positive charge) interacts with the electron (negative charge).
Coulomb’s Law Equation:
| F = | Q q1 q2 |
| r2 |
F is the force, how strong / tight / close the electron is held to the proton. Big Q is a constant (ignore it) The q1 is the amount of protons. The q2 is the amount of electrons. The r is the distance between the proton and electron and is squared in the equation. Coulomb’s Law essentially tells us that protons and electrons are held together in an atom by a combination of the distance between the protons and electrons and how many protons and electrons they have.
If you really want to understand the periodic trends and this fundamental law of nature (Coulomb’s Law), then you need to focus on 2 things:
1) The distance between protons and the electrons is the most important factor of the periodic trends. Particularly the distance of the outer most electrons to the protons. The shorter the distance between the outermost electrons and the nucleus, the more tightly it is bound to the atom. Therefore, shorter distance means higher electronegativity, higher ionization energy, and smaller atomic radius.
2) How many protons and neutrons an atom has is a less important factor of the periodic trends than the distance. The more protons and electrons an element has means higher electronegativity, higher ionization energy, and smaller atomic radius.
Examples: Use the logic of Coulomb’s Law to determine the differences in the periodic trend. Use the periodic table if you need it. VIDEO Determining Periodic Trend by Coulomb’s Law Examples Video 1.
1) Which element has a HIGHER electronegativity and why? Nitrogen or Sulfur.
Answer: Nitrogen because its outer electrons are closer to the nucleus.
2) Which element has a SMALLER atomic radius? Aluminum or Silicon.
Answer: Silicon because it has 1 more proton than Aluminum but their outer most electrons are about the same distance from the nucleus.
3) Which element has a LOWER ionization energy? Germanium or Arsenic.
Answer: Germanium because it has 1 less proton than Arsenic but their outer most electrons are about the same distance from the nucleus.
4) Which element has a LARGER atomic radius and why? Phosphorus or Selenium.
Answer: Selenium because its outer electrons are further from the nucleus.
PRACTIC PROBLEMS: Use the logic of Coulomb’s Law to determine the differences in the periodic trend. Use the periodic table if you need it.
1) Which element has a HIGHER electronegativity and why? Xenon or Bromine.
Answer: Bromine because its outer electrons are closer to the nucleus.
2) Which element has a SMALLER atomic radius? Magnesium or Sodium.
Answer: Magnesium because it has 1 more proton than Sodium but their outer most electrons are about the same distance from the nucleus.
3) Which element has a HIGHER ionization energy? Chlorine or Oxygen.
Answer: Oxygen because its outer electrons are closer to the nucleus.
4) Which element has a LARGER atomic radius? Rubidium or Strontium.
Answer: Rubidium because it has 1 less proton than Strontium but their outer most electrons are about the same distance from the nucleus.
Chem – College: Ionic Radius (Ionic Size)
How do you compare the ionic radius (ionic size) of two or more atoms?
Ionic radius is really the same as atomic radius with some modifications. Here the ionic radius table is not very important for solving possible problems you will encounter on a test, but it does give you a good idea of how the atomic radius changes as you take a non-charged element and turn it into an ion. The ionic radius describes how the radius or size of an atom changes when electrons are added or taken away to form an ion. The general rule for ionic radius is that as you add electrons the ionic radius gets bigger and as you take way electrons the ionic radius gets smaller. Therefore:
O2- is larger that O
Ca2+ is smaller than Ca
However, it can be more complicated than that. If there is no difference in the amount of electrons (if they are isoelectric) then you have to look to see if you have a difference in the amount of protons. The more protons an atom or ion has the smaller it is. The less protons an atom or ion has the larger it is. Therefore:
Na+ is smaller than N3-
Both have the same number of electrons = 10
But they have different amounts of protons
Na = 11 N = 7
What you want to always keep in mind for ionic radius is that you should count up the electrons and protons in every case and then compare it to each other. Remember, electrons are the main reason that atoms are bigger so they are more important but if the electron numbers are the same then look at the proton numbers.
Examples: Put the Ions below in order from LARGEST to SMALLEST.
| Ions | Largest to Smallest |
| S2-, Cl–, Si4-, P3- | Si4->P3->S2->Cl– |
| Be2+, B3+, Li+, C4+ | Li+>Be2+>B3+>C4+ |
| F–, Na+, Se2-, Sr2+ | Se2->Sr2+>F–>Na+ |
VIDEO Ionic Radius Demonstrated Example 1: Put the ions below in order form LARGEST to SMALLEST.
Rb+, As3-, K+, S2-
How many electrons do each of them have?
Rb = 36
As = 36
K = 18
S = 18
So we see that we have two groups. The group with more electrons will be larger. So far we know that Rb and As will be larger than K and S. To find out more we look at the protons.
How many protons do each have?
Rb = 37
As = 33
K = 19
S = 16
Since electrons contribute more to the size, we look at them first. Rb and As have 36 electrons. Which one has less protons?
As….Therefore As is the largest and Rb is the next largest.
Between K and S which both have 18 electrons which one has more protons?
K….Therefore K is the smallest and S is the next smallest.
COMPLETE ANSWER: As3->Rb+>S2->K+
PRACTICE PROBLEMS: Put the ions below in order from SMALLEST to LARGEST using just a regular periodic table.
| Ions | Smallest to Largest |
| N3-, Mg2+, F–, Na+ | Mg2+<Na+<F–<N3- |
| P3-, Cl–, Cs+, I– | Cl–<P3-<Cs+<I– |
| Te2-, Br–, Ba2+, Sr2+ | Sr2+<Br–<Ba2+<Te2- |
Chem – Atomic Radius
What is the atomic radius?
For this section, open up your atomic radius periodic table with this link. The atomic radius means the size of an atom. Since the atom is a circle and the length of a radius of a circle determines its size, this is why they call the size of the atom the atomic radius. There are two factors that determine the atomic radius. First is the amount of electrons or electron shells it has. The more electrons an element has the larger it is. This is why the atomic radius increases as you go down the periodic table. The second factor in determining atomic radius is the number of protons an element has. Since the protons are positively charged, they pull the electrons to them and thus shrink the outer edge of the electrons, shrinking the whole atom. This is why the atomic radius is larger on the left side of the periodic table and smaller on the right.
Keep in mind the number of electrons is more important than the number of protons. You only take into account the number of protons when they number of electrons between different elements is very close in number. This happens when the atomic radius of the elements you are talking about are in the same electron shell (electron shells on the periodic table). Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend. You may notice that the trend is the opposite of electronegativity and ionization energy.
Try a quick sketch of the periodic table with the atomic radius trend at least 3 times to help memorization before you go on to the next example and practice problems.
Like electronegativity and ionization energy questions are usually asked where they give you about 4 elements and they tell you to pick out the element with the largest or smallest atomic radius.
Examples: Without looking at an atomic radius trend (but you can use a regular periodic table), pick out the element with the LARGEST atomic radius. If necessary you can refer to the atomic radius periodic table. VIDEO Comparing Atomic Radius Between Elements Examples 1.
| Li, Rb, Cs, Na | Cs |
| Fe, Ca, Br, Zn | Ca |
| Co, Si, O, Sr | Sr |
PRACTICE PROBLEMS: Without looking at an atomic radius trend (but you can use a regular periodic table), pick out the element with the SMALLEST atomic radius. If necessary you can refer to the atomic radius periodic table.
| Cl, I, At, Br | Cl |
| Al, Mg, S, Na | S |
| Te, O, Se, Po | O |
| Xe, Ar, He, Ne | He |
| P, Mn, K, Ga | P |
| Hg, Fr, F, N | F |
Chem – Ionization Energy
What is ionization energy?
For this section, you can open up your ionization energy table with this link. Ionization energy is another of the periodic trends. Ionization energy is the amount of energy it takes to remove an electron from the atom of an element. When they mention this, they are usually talking about the outermost electron or they will call it the first ionization energy. Although that is a decent definition, I prefer to think of ionization energy as how difficult it is to remove or how hard do you need to pull in order to remove an electron from the atom of an element. If you imagine that you were able to physically grab just one electron of an element, would you need to pull it really hard or could you just pop it off with a single finger? If you need to pull the electron really hard to remove it from the element that means it has a high ionization energy. Conversely, if the electron is easy to pull off that means it has a low ionization energy.
The value (number) for the ionization energy of each element is not really important. You will rarely be asked anything about the values of ionization energy. Instead, most questions will be focused on what is the overall, or general trend, of ionization energy. One trend is ionization energy generally increases as we go from the bottom to the top of the periodic table. It is low at the bottom and high on top. The other trend is ionization energy generally increases as we go from left to right. It is very low on the left side of the periodic table and very high on the right side. Overall, ionization energy is low in the bottom left and high on the top right of the periodic table. If we compare this to electronegativity, we see that the trend is basically the same. The only difference is ionization energy includes the noble gases (group 18) elements whereas, electronegativity does not.Like electronegativity, ionization energy questions are usually asked where they give you about 4 elements and they tell you to pick out the element with the lowest or highest ionization energy. Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend.
Try a quick sketch of the periodic table with the ionization energy trend at least 3 times to help memorization before you go on to the example and practice problems.
Examples: Without looking at an ionization energy trend (but you can use a regular periodic table), pick out the element with the LOWEST ionization energy. If necessary you can refer to the ionization energy table with this link. VIDEO Comparing Ionization Energy Between Elements Examples 1.
| Ba, Au, Pb, At | Ba |
| N, Sb, As, P | Sb |
| Ag, Si, F, Ca | Ca |
PRACTICE PROBLEMS: Without looking at an ionization energy trend (but you can use a regular periodic table), pick out the element with the HIGHEST ionization energy. If necessary you can refer to the ionization energy table with this link.
| Mg, Cl, Ar, Al | Ar |
| Cr, Se, Ca, Ge | Se |
| Be, Sr, Ba, Mg | Be |
| Br, I, At, F | F |
| Fe, Cs, S, Ni | S |
| Ag, O, Si, Na | O |
Chem – Electronegativity Part 2
What is the trend for electronegativity?
If you look at the electronegativity table carefully, you can also see a trend. Since the noble gases ( group 18 ) have been eliminated from this table, if you look from left to right you will notice that the numbers generally increase as you further to the right side of the table. We can say electronegativity generally increases as we go from left to right on the table. Just like the left to right trend, we can also create a trend that goes up and down. If we start from the bottom of the electronegativity table and go up, we can see the number generally increases as we get closer to the top of the table. We can say electronegativity generally increases as we go from bottom to top on the table. If we combine these two trends of left to right and down to up, then we get an overall trend. As we go from the bottom left to the top right, electronegativity generally increases. Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend.
Try a quick sketch of the periodic table with the electronegativity trend at least 3 times to help memorization before you go on to the next example and practice problems.
Another way questions about electronegativity can be asked is to compare two or more elements to each other and ask which has the highest or lowest electronegativity. To answer these questions on a test, you will need to have the electronegativity trend memorized. You should always have access to a regular periodic table. The following examples are the most typical questions asked.
Examples: WITHOUT looking at an electronegativity table (but you can use a regular periodic table) pick out the element with the HIGHEST electronegativity. VIDEO Comparing Electronegativity Between Elements Examples 1.
| Fe, Ga, Ca, Se | Se |
| Cs, Li, K, Rb | Li |
| As, Ba, Cl, Ag | Cl |
PRACTICE PROBLEMS: WITHOUT looking at an electronegativity table (but you can use a regular periodic table) pick out the element with the HIGHEST electronegativity.
| C, N, F, Be | F |
| Te, Po, S, Se | S |
| Cr, As, Co, K | As |
| Ag, Sr, Sn, I | I |
| Zn, Ba, Mn, P | P |
| Au, O, Cs, Si | O |
Chem – Electronegativity Part 1
What is electronegativity?
For this section, open up your electronegativity periodic table with this link. Electronegativity is one of what they call the periodic trends. Electronegativity is the ability of an atom of an element to pull electrons to it. The higher the electronegativity of the element, the more likely it is to pull an electron to it. Although most people will not describe electronegativity like I do, the definition I give is extremely simple, powerful, and useful compared to most definitions of electronegativity. The numbers on your electronegativity table run from 0 to 4. 0 is the lowest electronegativity and 4 is the highest electronegativity. An easy way to think about electronegativity is; if you have two atoms of different elements, like carbon and fluorine, and you put an electron exactly between them. Which element does it get pulled to? It gets pulled to fluorine because flourine has a higher electronegativity. One way questions about electronegativity can be asked is very direct like the questions below.
Examples: While looking at your electronegativity table, give the electronegativity of each element.
| P | 2.2 |
| Ca | 1.3 |
| H | 2.1 |
PRACTICE PROBLEMS: While looking at your electronegativity table, give the electronegativity of each element.
| Na | 0.9 |
| S | 2.5 |
| Ba | 0.9 |
| Al | 1.6 |
Chem – Drawing Valence Electrons Around an Atom
What sections should I know before attempting to learn this section?
How do you display valence electrons around an atom or element?
Now that we know how to count the valence electrons we should learn the most common way to display them. This method is known as the valence electron dot method. It involves writing the atom or element you want to display as it’s letter symbol and then placing the valence electrons around it as dots.
Examples: Draw the valence electron dot representation of the elements below. Look at your valence electron periodic table while figuring these out. VIDEO Drawing Valence Electrons Around Atoms Examples 1.
What is the valence electron dot representation of Beryllium?
Answer:
What is the valence electron dot representation of Nitrogen?
Answer:
What is the valence electron dot representation of Chlorine?
Answer:
PRACTICE PROBLEMS: Draw the valence electron dot representation of the elements below. Look at your valence electron periodic table while figuring these out.
What is the valence electron dot representation of Sodium?
What is the valence electron dot representation of Carbon?
What is the valence electron dot representation of Neon?
What is the valence electron dot representation of Aluminum?
What is the valence electron dot representation of Calcium?
Chem – Valence Electrons
What sections should I know before attempting to learn this section?
What are valence electrons?
Valence electrons are the outermost electrons of the element. This overlaps with the electron shells which were described in the previous section. In fact, in many cases (but not all) the amount of electrons in the last electron shell are the same as the valence electrons. The valence electrons simply do not include the periodic groups of 3 through 12. Most people will call these groups the transition metals. When you are figuring out the valence electrons, you simply leave out groups 3 through 12. This is a periodic table link that shows the valance electrons organized on the periodic table. Below is a table of how the valence electrons match up with the different periodic table groups.
| Group | Valence Electrons |
| Group 1 | 1 |
| Group 2 | 2 |
| Group 13 | 3 |
| Group 14 | 4 |
| Group 15 | 5 |
| Group 16 | 6 |
| Group 17 | 7 |
| Group 18 | 8 |
Examples: How many valence electrons do these elements tend to have? Look at your valence electron periodic table while figuring these out.
| C | 4 |
| Br | 7 |
| Ca | 2 |
VIDEO Valence Electrons Demonstrated Example 1: How many valence electrons does nitrogen have?
Which periodic table row is nitrogen in?
Answer: 2nd
Start counting boxes across from the first element on the second row (Li) until you get to nitrogen. How many boxes did you count?
Answer: 5
So how many valence electrons does nitrogen have?
COMPLETE ANSWER: 5 valence electrons
PRACTICE PROBLEMS: How many valence electrons do these elements tend to have? Use the periodic table to solve. If needed use the valence periodic table.
| K | 1 |
| Se | 6 |
| Mg | 2 |
| N | 3 |
| Cs | 1 |
| I | 7 |
Chem – Bohr Model and Electron Shells Part 2
What sections should I know before attempting to learn this section?
—> Bohr Model and Electron Shells Part 1
How many electrons are in the last electron shell of an element?
In the previous section we described how the Bohr Model relates to the amount of electron shells of an element. Now we are going to ask the more detailed question from above.
Examples: How many electrons are in the last electron shell of each pure element? Use the electron shells periodic table to solve.
| He | 2 |
| C | 4 |
| S | 6 |
VIDEO Electron Shell Demonstrated Example 1: How many electrons are in the last electron shell of pure Si? Use the electron shells periodic table to solve.
What period (row) is Si in?
Answer: 3
With what element does period 3 start with?
Answer: Na
How many elements do I have to count including Na until I get to Si on period 3?
Answer: 4
So how many electrons does Si have in its last electron shell?
COMPLETE ANSWER: 4 electrons in the last electron shell
PRACTICE PROBLEMS: How many electrons are in the last electron shell of each pure element? Use the electron shells periodic table to solve.
| Cl | 7 |
| Ne | 8 |
| Al | 3 |
| Be | 2 |
| B | 3 |
| O | 6 |
Chem – Bohr Model and Electron Shells Part 1
What sections should I know before attempting to learn this section?
What is the Bohr model of the atom?
The amount and arrangement of electrons around two or more different atoms are the best way to predict how they will interact. This is why electrons are talked about so extensively in chemistry. The most simplistic, yet still correct way to understand how electrons move in atoms is called the Bohr model. The Bohr model is simply a picture of the atom with the protons and neutrons in the middle and the electrons traveling in ever-larger rings around that middle. Some people call this the solar system model because it looks much like our solar system with the sun (nucleus) in the middle and the planets (electrons) running in ever-wider rings around the center. Here is a picture of the Bohr model.
The rings that electrons travel in the Bohr model are referred to as electron shells. The first electron shell is closest to the nucleus and the second shell a little further away. As we get higher and higher in electron shells we get further from the nucleus. From the periodic table, you can model how the electron shells are created. This is a link of the electron shells shown on the periodic table. The picture above of the Bohr model is a representation of an atom with the first three energy shells full of electrons. It would therefore be representing the neutral atom of Argon (Keep in mind the nucleus is not completely drawn there).
In chemistry classes, two common questions are asked about the electrons shells:
1) How many electron shells does a certain element have?
2) How many electrons are in the last electron shell of a particular element?
We answer the first question in this section and the second question in the next section.
Examples: How many electron shells does each pure element have? Use the electron shells periodic table. VIDEO Electron Shells Examples 1.
| Li | 2 |
| P | 3 |
| I | 5 |
PRACTICE PROBLEMS: How many electron shells does each pure element have? Try using the regular periodic table. Use the electron shells periodic table if you need to.
| Sn | 5 |
| Ba | 6 |
| Cu | 4 |
| N | 2 |
Chem – Further Divisions of the Periodic Table
By far the most important division is the metals versus the non-metals, which we just talked about. However, some people also like to talk about how the periodic table is broken up into smaller divisions. The most common ones are alkali metals, alkaline earth metals, transition metals, metalloids, halogens, noble gasses, lanthanoids, and actinoids. This periodic table is very good at showing you where they are. The alkali metals are those in group number 1 from lithium down. Remember hydrogen is not a metal so it cannot be counted in that category. The alkaline earth metals follow group 2 from beryllium down. The transition metals include groups 3 through 12 starting with the column scandium and ending with the column of zinc. For the transition metals, some teachers will include elements beyond group 12 like tin and lead. Either way, they should make this clear, but many of them do not. The metalloids can also be a confusing category. They roughly run along the step-stair division between the metals and non-metals. They include B, Si, Ge, As, Sb, Te, and Po. Note that aluminum is not usually included in the metalloid category. The halogens are group 17, from fluorine down. The noble gasses are group 18, from helium down.
PRACTICE PROBLEMS: Name at least one element in that division or give the division that the element is in (Use the periodic table link in the text above to guide you).
| Transition metals | Cr, Mn, Fe, Co, Ni, Pt, Cu, Ag, Au, Zn… |
| Halogens | F, Cl, Br, I, At, Uus |
| Alkali metals | Li, Na, K, Rb, Cs, Fr |
| Alkali earth metals | Barium |
| Transition metals | Mercury |
| Noble gasses | Neon |