Scientific Tutor
Chem – Electronegativity Part 2
What is the trend for electronegativity?
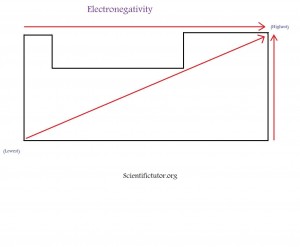
If you look at the electronegativity table carefully, you can also see a trend. Since the noble gases ( group 18 ) have been eliminated from this table, if you look from left to right you will notice that the numbers generally increase as you further to the right side of the table. We can say electronegativity generally increases as we go from left to right on the table. Just like the left to right trend, we can also create a trend that goes up and down. If we start from the bottom of the electronegativity table and go up, we can see the number generally increases as we get closer to the top of the table. We can say electronegativity generally increases as we go from bottom to top on the table. If we combine these two trends of left to right and down to up, then we get an overall trend. As we go from the bottom left to the top right, electronegativity generally increases. Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend.
Try a quick sketch of the periodic table with the electronegativity trend at least 3 times to help memorization before you go on to the next example and practice problems.
Another way questions about electronegativity can be asked is to compare two or more elements to each other and ask which has the highest or lowest electronegativity. To answer these questions on a test, you will need to have the electronegativity trend memorized. You should always have access to a regular periodic table. The following examples are the most typical questions asked.
Examples: WITHOUT looking at an electronegativity table (but you can use a regular periodic table) pick out the element with the HIGHEST electronegativity. VIDEO Comparing Electronegativity Between Elements Examples 1.
| Fe, Ga, Ca, Se | Se |
| Cs, Li, K, Rb | Li |
| As, Ba, Cl, Ag | Cl |
PRACTICE PROBLEMS: WITHOUT looking at an electronegativity table (but you can use a regular periodic table) pick out the element with the HIGHEST electronegativity.
| C, N, F, Be | F |
| Te, Po, S, Se | S |
| Cr, As, Co, K | As |
| Ag, Sr, Sn, I | I |
| Zn, Ba, Mn, P | P |
| Au, O, Cs, Si | O |