Scientific Tutor
Chem – Atomic Radius
What is the atomic radius?
For this section, open up your atomic radius periodic table with this link. The atomic radius means the size of an atom. Since the atom is a circle and the length of a radius of a circle determines its size, this is why they call the size of the atom the atomic radius. There are two factors that determine the atomic radius. First is the amount of electrons or electron shells it has. The more electrons an element has the larger it is. This is why the atomic radius increases as you go down the periodic table. The second factor in determining atomic radius is the number of protons an element has. Since the protons are positively charged, they pull the electrons to them and thus shrink the outer edge of the electrons, shrinking the whole atom. This is why the atomic radius is larger on the left side of the periodic table and smaller on the right.
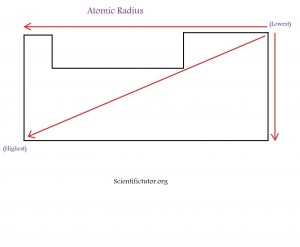
Keep in mind the number of electrons is more important than the number of protons. You only take into account the number of protons when they number of electrons between different elements is very close in number. This happens when the atomic radius of the elements you are talking about are in the same electron shell (electron shells on the periodic table). Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend. You may notice that the trend is the opposite of electronegativity and ionization energy.
Try a quick sketch of the periodic table with the atomic radius trend at least 3 times to help memorization before you go on to the next example and practice problems.
Like electronegativity and ionization energy questions are usually asked where they give you about 4 elements and they tell you to pick out the element with the largest or smallest atomic radius.
Examples: Without looking at an atomic radius trend (but you can use a regular periodic table), pick out the element with the LARGEST atomic radius. If necessary you can refer to the atomic radius periodic table. VIDEO Comparing Atomic Radius Between Elements Examples 1.
| Li, Rb, Cs, Na | Cs |
| Fe, Ca, Br, Zn | Ca |
| Co, Si, O, Sr | Sr |
PRACTICE PROBLEMS: Without looking at an atomic radius trend (but you can use a regular periodic table), pick out the element with the SMALLEST atomic radius. If necessary you can refer to the atomic radius periodic table.
| Cl, I, At, Br | Cl |
| Al, Mg, S, Na | S |
| Te, O, Se, Po | O |
| Xe, Ar, He, Ne | He |
| P, Mn, K, Ga | P |
| Hg, Fr, F, N | F |