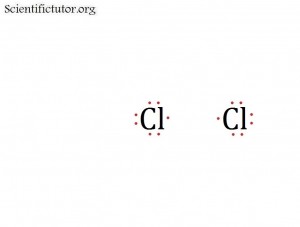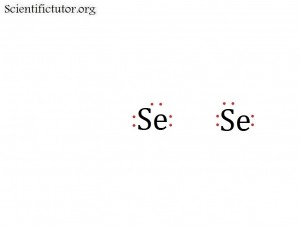Scientific Tutor
Archive for the ‘Chemistry’ Category
Chem – How to Convert Between Grams and Atoms
The last step we are going to address in this particular conversion map is the conversion to atoms. Converting between atoms and molecules is much easier than the steps that we have done before. However, it seems to many students because of that they get thrown off and have a difficult time. This conversion is all about how many of each element are in a particular substance. Check out the examples below for a more detailed explanation.
If we wanted to say how many atoms of Br are in NBr3 then we would say 3. Now the only difference with these conversions is we have to turn that statement into a ratio between the molecule (NBr3) and the atom (Br).
| 1 NBr3 |
| 3 Br |
or
| 3 Br |
| 1 NBr3 |
We can also do this for molecules that have only one atom of one element. It just looks kind of silly so most teachers and books will not show it to you. Lets say we had the molecule Ni. How many atoms of Ni are in the molecule of Ni? 1 atom of Ni for every 1 molecule of Ni.
| 1 Ni |
| 1 Ni |
or
| 1 Ni |
| 1 Ni |
Now the above may look trivial and painfully obvious but believe it or not a lot of students run into problems with the above ratio and it is nothing to be ashamed of. It is hard for people to describe and therefore it is hard for students to understand correctly. In these special cases where there is only 1 atom per 1 molecule then you don’t really need to do any extra math steps. However, I will show you to them in my demonstrated examples to make sure everyone understands what is going on.
This last step in our conversion map is the arrow furthest to the right (the purple text).
VIDEO Converting Between Grams and Atoms Demonstrated Example 1: If you have 12g of CH4 how many atoms of hydrogen do you have? You will need the periodic table for this question.
Step 1:
What information does the problem give you?
Answer: 12g CH4
Step 2:
What units does the question ask for?
Answer: ? atoms H
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 3 arrows when we go from Grams —> Moles —> Molecules —> Atoms. 3 arrows = 3 conversion
Step 4:
How do we set up the problem?
Answer: First box is info given, next 3 boxes are the 3 conversion, last box (fifth box) is what the question asked for.
| 12 g CH4 | atoms H | |||
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of CH4 found on the periodic table
Step 6:
What is the molar mass of CH4?
Answer: about 16 g/ 1 mol
Step 7:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 12 g CH4 | 1 mol | atoms H | ||
| 16 g |
Step 8:
What conversion should we use next?
Answer: Avogadro’s number (moles to molecules ratio) found on the conversion map. 6.022 * 1023 molecules / 1 mol
| 12 g CH4 | 1 mol | 6.022 * 1023 molec | atoms H | |
| 16 g | 1 mol |
Step 9:
What conversion should I use next?
Answer: the atom to molecule ratio (4 H to 1 CH4)
| 12 g CH4 | 1 mol | 6.022 * 1023 molec | 4 atoms H = | atoms H |
| 16 g | 1 mol | 1 molec CH4 |
Step 10:
How do I know when I am done with the conversions?
Answer: All other units but the ones in the answer are crossed out through cancellation. For this example atoms and H remain not crossed out.
| 12 g CH4 | 1 mol | 6.022 * 1023 molec | 4 atoms H = | atoms H |
| 16 g | 1 mol | 1 molecCH4 |
Step 11: Simplify
| 12 | 1 | 6.022 * 1023 | 4 atoms H = | atoms H |
| 16 | 1 | 1 |
Step 12:
How do I calculate?
Answer: (12 * 6.022 * 1023 *4) / (16) = 1.81 * 1024
Step 13:
COMPLETE ANSWER: 1.81 * 1024 atoms of H
VIDEO Converting Between Grams and Atoms Demonstrated Example 2: 6.4 * 1025 atoms of Fe is how many grams of Fe? You will need the periodic table for this question.
UNFORTUNATELY I AM NOT ABLE TO SHOW THIS DEMONSTRATED EXAMPLE ABOVE IN TEXT BECUASE THERE IS NOT ENOUGH ROOM ON THE WEBPAGE.
PRACTICE PROBLEMS: Complete grams and atoms conversions. Make sure you have this periodic table link open when answering these questions and use the conversion map if you need it.
How many F atoms are in 20g of CaF2?
Answer: 3.08 * 1023 atoms F
If you have 62 grams of Chromium how many atoms is that?
Answer: 7.18 * 1023 atoms Cr
If you have 7.89 * 1025 atoms of Boron how many grams is that?
Answer: 1.415 * 103 g B …or… 1415 g B
How many grams of Li2S can you make from 1.3 * 1024 atoms of Li?
Answer: 49.6 g Li2S
Chem – How to Convert Between Grams and Molecules
Now we explore the next step in our conversion map. This step will help us explain the definition of a mole. A mole is something that is hard to understand at first. Quite simply, a mole is just a number of objects in this case atoms or molecules. Therefore, a mole is an amount of something. The best analogy to a mole is a dozen. A dozen is an amount of something, specifically 12 of them. The only difference between a dozen and a mole is that the number or amount of things is different. A mole is 6.022 * 1023 things, whereas a dozen is 12 things. So this is where we get the conversion between 1 mole and 6.022 * 1023 molecules.
| 6.022 * 1023 molecules |
| 1 mol |
or
| 1 mol |
| 6.022 * 1023 molecules |
This ratio is seen on the right arrow (the green text) in the conversion map below.
On a side note, I tend to abbreviate the units of molecules as ( molec ) for reasons of saving space and to help distinguish it from moles which is something different.
VIDEO Converting Between Grams and Molecules Demonstrated Example 1: If you have 5.4g of Cr then how many molecules of Cr would you have? You will need the periodic table for this question.
Step 1:
What information are we given?
Answer: 5.4 g Cr
Step 2:
What units does the question ask for?
Answer: ? molecules Cr
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 2 arrows when we go from Grams —> Moles —> molecules. 2 arrows = 2 conversions
Step 4:
How do we set up the problem?
Answer: First box is info given, next two boxes are the conversions, last box (fourth box) is what the question asked for
| 5.4 g Cr | Molecules Cr | ||
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of Chromium found on the periodic table
Step 6:
What is the molar mass of Cr?
Answer: about 52 g/ 1 mol
Step 7:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 5.4 g Cr | mol | molec Cr | |
| g |
Step 8:
What comes next?
Answer: fill in the numbers and cross out units
| 5.4 g Cr | 1 mol | molec Cr | |
| 52 g |
Step 9:
Simplify by removing all crossed out units.
| 5.4 Cr | 1 mol | molec Cr | |
| 52 |
Step 10:
What is the next conversion?
Answer: Avogadro’s number (moles to molecules ratio) found on the conversion map. 6.022 * 1023 molecules / 1 mol
Step 11:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 5.4 Cr | 1 mol | molec = | molec Cr |
| 52 | mol |
Step 12:
What comes next?
Answer: fill in the numbers and cross out units
| 5.4 Cr | 1 mol | 6.022 * 1023 molec = | molec Cr |
| 52 | 1 mol |
Step 13:
Simplify by removing all crossed out units.
| 5.4 Cr | 1 | 6.022 * 1023 molec = | molec Cr |
| 52 | 1 |
Step 14:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case molec and Cr.
| 5.4 Cr | 1 | 6.022 * 1023 molec = | molec Cr |
| 52 | 1 |
Step 15:
How do I do the calculations?
Answer: (5.4 * 6.022 * 1023) / (52) = 6.25 * 1022
| 5.4 Cr | 1 | 6.022 * 1023 molec = | 6.25 * 1022 molec Cr |
| 52 | 1 |
Step 16:
COMPLETE ANSWER: 6.3 1023 molecules of Cr
VIDEO Converting Between Grams and Molecules Demonstrated Example 2: When you have 7.4 * 1025 molecules of O2 how many grams is that? You will need the periodic table for this question.
Step 1:
What information are we given?
Answer: 7.4 * 1025 molecules O2
Step 2:
What units does the question ask for?
Answer: ? g O2
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 2 arrows when we go from Molecules —> Moles —> Grams. 2 arrows = 2 conversions
Step 4:
How do we set up the problem?
Answer: First box is info given, next two boxes are the conversions, last box (fourth box) is what the question asked for
| 7.4 * 1025 molec O2 | g O2 | ||
| 1 |
Step 5:
What is the first conversion?
Answer: Avogadro’s number (moles to molecules ratio) found on the conversion map. 6.022 * 1023 molecules / 1 mol
Step 6:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 7.4 * 1025 molec O2 | mol | g O2 | |
| molec |
Step 7:
What comes next?
Answer: fill in the numbers and cross out units
| 7.4 * 1025 molec O2 | 1 mol | g O2 | |
| 6.022 * 1023 molec |
Step 8:
Simplify by removing all crossed out units.
| 7.4 * 1025 O2 | 1 mol | g O2 | |
| 6.022 * 1023 |
Step 9:
What is the next conversion?
Answer: molar mass (grams to mole ratio) of O2 found on the periodic table
Step 10:
What is the molar mass of O2?
Answer: about 32 g/ 1 mol
Step 11:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 7.4 * 1025 O2 | 1 mol | g = | g O2 |
| 6.022 * 1023 | mol |
Step 12:
What comes next?
Answer: fill in the numbers and cross out units
| 7.4 * 1025 O2 | 1 mol | 32 g = | g O2 |
| 6.022 * 1023 | 1 mol |
Step 13:
Simplify by removing all crossed out units.
| 7.4 * 1025 O2 | 1 | 32 g = | g O2 |
| 6.022 * 1023 | 1 |
Step 14:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case g and O2.
| 7.4 * 1025 O2 | 1 | 32 g = | g O2 |
| 6.022 * 1023 | 1 |
Step 15:
How do I do the calculations?
Answer: (7.4 * 1025 * 32) / (6.022 * 1023) = 3932
| 7.4 * 1025 O2 | 1 | 32 g = | 3932 g O2 |
| 6.022 * 1023 | 1 |
Step 16:
COMPLETE ANSWER: 3900 g of O2
PRACTICE PROBLEMS: Solve the conversions between grams and molecules. Make sure you have this periodic table link open when answering these questions. You may also need the conversion map. Answers are rounded to the nearest significant figure.
How many molecules are in 16g of CO2?
Answer: 2.2 * 1023 molec CO2
If you have 3.0 * 1024 molecules of K2S how many grams is that?
Answer: 55 g K2S
How many molecules are in 70g of Sn?
Answer: 3.5 * 1023 molecules Sn
If you have 9.2 * 1020 molecules of Cl how many grams is that?
Answer: 0.053 g Cl
Chem – How to Convert Between Grams and Moles
Some of you may have noticed that the molar mass (or atomic mass) can be written in terms of a ratio. This means we can use it as a conversion. If your molar mass of CH4 is 16 g/mol that means we can write it as a ratio like the ones below.
| 16 g |
| 1 mol |
or
| 1 mol |
| 16 g |
Either way, both are true statements of the relationship between grams and moles of CH4. That means you can write the molar mass for a conversion whichever way you need to arrange the units correctly. For all conversions going forward we are going to start using conversion maps to help guide us. Right now some people may feel they are a waste of time. However, as chemistry gets more complex so do the conversion maps. Our first conversion map is how to go between grams and moles. It is the map below.
On a side note, I tend to abbreviate the units of moles as ( mol ) for reasons of saving space and to help distinguish it from molecule which is something different.
VIDEO Converting Between Grams and Moles Demonstrated Example 1: If we have 12 g of He how many moles of He is that? You will need the periodic table for this question.
Step 1:
What information are we given?
Answer: 12g He
Step 2:
What units does the question ask for?
Answer: ? mol He
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 1 arrow when we go from Grams —> Moles. 1 arrows = 1 conversion
Step 4:
How do we set up the problem?
Answer: First box is info given, second box is the 1 conversion, last box (third box) is what the question asked for.
| 12g He | mol He | |
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of Helium found on the periodic table
Step 6:
What is the molar mass of He?
Answer: about 4 g/ 1 mol
Step 7:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 12g He | mol | mol He |
| g |
Step 8:
What comes next?
Answer: fill in the numbers and cross out units
| 12g He | 1 mol = | mol He |
| 4 g |
Step 9:
Simplify by removing all crossed out units.
| 12 He | 1 mol = | mol He |
| 4 |
Step 10:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case mol and He
| 12 He | 1 mol = | 3 mol He |
| 4 |
Step 11:
How do I do the calculations?
Answer: 12 * 1 / 4 = 3
| 12 He | 1 mol = | 3 mol He |
| 4 |
COMPLETE ANSWER: 3.0 mol He
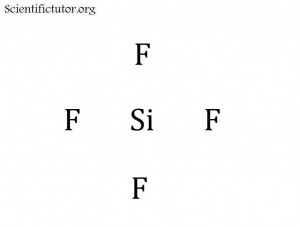
VIDEO Converting Between Grams and Moles Demonstrated Example 2: How many grams of SiH4 is 3.7 moles of SiH4? You will need the periodic table for this question.
Step 1:
What information are we given?
Answer: 3.7 mol SiH4
Step 2:
What units does the question ask for?
Answer: ? g SiH4
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 1 arrow when we go from Moles —> Grams. 1 arrows = 1 conversion
Step 4:
How do we set up the problem?
Answer: First box is info given, second box is the 1 conversion, last box (third box) is what the question asked for
| 3.7 mol SiH4 | g SiH4 | |
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of Aluminum found on the periodic table
Step 6:
What is the molar mass of SiH4?
Answer: about 32 g/ 1 mol
Step 7:
How do we set that up in the conversion?
Answer: units first, set up the units that need to cancel out (in red)
| 3.7 mol SiH4 | g = | g SiH4 |
| mol |
Step 8:
What comes next?
Answer: fill in the numbers and cross out units
| 3.7 mol SiH4 | 32 g = | g SiH4 |
| 1 mol |
Step 9:
Simplify by removing all crossed out units.
| 3.7 SiH4 | 32 g = | g SiH4 |
| 1 |
Step 10:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case mol and SiH4
| 3.7 SiH4 | 32 g = | g SiH4 |
| 1 |
Step 11:
How do I do the calculations?
Answer: 3.7 * 32 = 118.4
| 3.7 SiH4 | 32 g = | 118 g SiH4 |
| 1 |
COMPLETE ANSWER: 120 g SiH4
PRACTICE PROBLEMS: Solve the conversions between grams and moles. Make sure you have this periodic table link open when answering these questions and also the conversion map. Answers are rounded to correct number of significant figures.
If you have 4.5 moles of potassium how many grams is that?
Answer: 180 g K
If you have 0.32 grams of silicon how many moles is that?
Answer: 0.011 mol Si
Given 8.2 moles of Ar how many grams will you have?
Answer: 330 g Ar
Given 9.7 grams of Ca how many moles is that?
Answer: 0.24 mol Ca
If you have 4.6 grams of NH3 how many moles is that?
Answer: 0.27 mol NH3
Given 1.2 mol of CaF2 how many grams would you have?
Answer: 94 g CaF2
Chem – LESSON 11: Grams, Moles, Molecules, and Atoms Conversions
What is the lesson about?
This lesson is about how to convert between the different measurements that are very commonly used in chemistry. Just like in your daily life, you convert between common things like time and distance. How long does it take you to get the class or work? Well, if it is 15 miles away then it may take you 20 minutes to get there. Now if you ask the question,” what time should you get up?” Then the 20 minutes is going to be a far more important measurement for you. However, if you are asking how much does each trip cost you then the 15 miles can be converted into a money amount that you can calculate. We do these different conversions every day and don’t notice it. In chemistry the same happens. The only difference is we are not used to the units of chemistry yet.
Why is it critical to understand?
If you have trouble with this chapter, then you are going to have trouble going forward in the chemistry class. Many of the units and questions displayed in this lesson are going to be added into later lessons like stoichiometry. Besides that, it is important to know for those people that make, use, or dispense things like drugs professionally. Drugs like aspirin are usually labeled on the bottle in grams or milligrams. This is in fact, not how you should measure their affects on your body. Their affects on your body are mostly determined by how many molecules are in the drug. So if you cannot convert between those two then you will not be able to safely make, use, or dispense that drug on a professional level. Other scientific fields like ecology (the study of the environment and its interactions with life) also can find significance either in how many of something you have or what is the total weight of the animals. Each situation is different and so to be able to convert between them allows you to sometimes find meaningful information.
What should you know before attempting this lesson?
It is strongly suggested that you know the sections of Introduction to Problem Solving, Unit Conversions, and Calculating the Molar Mass of Compounds before you start learning this lesson.
New Learning Sections:
—> How to Convert Between Grams and Moles
—> How to Convert Between Grams and Molecules
—> How to Convert Between Grams and Atoms
—> Empirical and Molecular Formula Explanation Part 1
—> Empirical and Molecular Formulas by Molar Mass Part 2
—> Empirical Formula from Mass Part 3
—> Empirical Formula from Percent Part 4
—> Empirical and Molecular Formula from Mass and Percent Part 5
Reference Pages:
—> Grams to Molecules Conversion Map
Worksheets:
—> Grams Moles Molecules Atoms Worksheet 1
—> Grams Moles Molecules Atoms Worksheet 1 WITH ANSWERS
Chem – Predicting Types of Chemical Equations Part 1
What sections should I know before attempting to learn this section?
IF YOU DO NOT FEEL CONFIDENT IN YOUR CHEMISTRY SKILLS SO FAR, I STRONGLY URGE YOU TO REVIEW THE DIFFERENT SECTIONS, LINKED IN BLUE, BELOW THIS TEXT.
2) Ions
5) Breaking Apart Ionic Compounds
6) Types of Chemical Equations
7) Diatomic Molecules in Chemical Equations
8) Balancing Chemical Equations
How do you predict the products of a chemical equation?
Predicting the products of a chemical equation is one of the most comprehensive and therefore complicated things we have had to do yet. Mostly, it centers around knowing how to take many concepts that we have learned in previous areas of chemistry and use them together to form one large picture of how the chemical reaction changes the compounds or molecules. In this section I left off the states of each of matter for each of the chemicals because I did not want that to distract people from understanding more important issues.
What are the most important steps to remember while you are trying to answer the practice problems about predicting chemical equations?
1) Remember to ALWAYS produce the chemical equation’s products BEFORE you try to balance how many of each chemical.
2) Some chemical equation types (mostly synthesis and decomposition) can have multiple different right answers for their products. Where that is possible I give only one right answer although more are possible. However, on single replacement, double replacement, and combustion the problems I picked have only one right answer and all those you encounter in your class should have only one right answer.
Examples: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Single Replacement:
Na + MgBr2 ——> ?
Answer: 2 Na + MgBr2 ——> Mg + 2 NaBr
Synthesis:
Ca + C4 + O2 ——> ?
Answer: 4 Ca + C4 + 6 O2 ——> 4 CaCO3
VIDEO Predicting Chemical Equations Demonstrated Example 1: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Single Replacement:
Li + Ba(NO3)2 ——> ?
Step 1:
Where do I start?
Answer: Look at the possible charges of each element according to the ions of the periodic table.
Li = +1, Ba = +2, NO3 = -1
Step 2:
How do I use the charges?
Both Li and Ba are positive charges so they will swap.
Step 3:
What is the compound made up of Li and NO3?
| Li+ | NO3– | |
| Total = | +1 | -1 |
Answer: LiNO3
Step 4:
What will Ba look like?
Answer: Since Ba is not a part of the diatomic molecules it will be single and alone: Ba
Step 5:
What does my answer look like so far?
Answer: Li + Ba(NO3)2 ——> Ba + LiNO3
Step 6:
How do I balance the equation?
COMPLETE ANSWER: 2 Li + Ba(NO3)2 ——> Ba + 2 LiNO3
VIDEO Predicting Chemical Equations Demonstrated Example 2: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Double Replacement:
MgO + KF ——> ?
Step 1:
Where do I start?
Answer: Look at the possible charges of each element according to the ions of the periodic table.
Mg = +2, O = -2, K = +1, F = -1
Step 2:
How do I use the charges?
Answer: The positive charges of Mg and K will swap places with their negative partners.
Step 3:
What is the compound made up of Mg and F?
If you are confused about this question and answer go back to the section how to form ionic compounds.
| Mg2+ | F– | |
| F– | ||
| Total = | +2 | -2 |
Answer: MgF2
Step 4:
What is the compound made up of K and O?
| K+ | O2- | |
| K+ | ||
| Total = | +2 | -2 |
Answer: K2O
Step 5:
What does my answer look like so far?
Answer: MgO + KF ——> MgF2 + K2O
Step 6:
How do I balance the equation?
If you are confused about this question and answer go back to an earlier section in this lesson called balancing chemical equations.
COMPLETE ANSWER: MgO + 2 KF ——> MgF2 + K2O
VIDEO Predicting Chemical Equations Demonstrated Example 3: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Single Replacement:
P + Fe2O3 ——> ?
Step 1:
Where do I start?
Answer: Look at the possible charges of each element according to the ions of the periodic table.
We have to break down the Fe and O compound to get the charge on Fe.
P = -3, Fe = +3, O = -2
| Fe3+ | O2- | |
| Fe3+ | O2- | |
| O2- | ||
| Total = | +6 | -6 |
Step 2:
How do I use the charges?
Both P and O are negative charges so they will swap.
Step 3:
What is the compound made up of Fe and P?
| Fe3+ | P3- | |
| Total = | +3 | -3 |
Answer: FeP
Step 4:
What will O look like?
Answer: Since O is a part of the diatomic molecules it will be paired with itself: O2
Step 5:
What does my answer look like so far?
Answer: P + Fe2O3 ——> FeP + O2
Step 6:
How do I balance the equation?
COMPLETE ANSWER:4 P + 2 Fe2O3 ——> 4 FeP + 3 O2
VIDEO Predicting Chemical Equations Demonstrated Example 4: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Double Replacement:
PbS2 + AlPO4 ——> ?
Step 1:
Where do I start?
Answer: Look at the possible charges of each element according to the ions of the periodic table.
We have to break down the Pb and S compound to get the charge on Pb.
Answer: Pb = +4, S = -2, Al = +3, PO4 = -3
| Pb4+ | S2- | |
| S2- | ||
| Total = | +4 | -4 |
Step 2:
How do I use the charges?
Answer: The positive charges of Pb and Al will swap places with their negative partners.
Step 3:
What is the compound made up of Pb and PO4?
| Pb4+ | PO43- | |
| Pb4+ | PO43- | |
| Pb4+ | PO43- | |
| PO43- | ||
| Total = | +12 | -12 |
Answer: Pb3(PO4)4
Step 4:
What is the compound made up of Al and S?
| Al3+ | S2- | |
| Al3+ | S2- | |
| S2- | ||
| Total = | +6 | -6 |
Answer: Al2S3
Step 5:
What does my answer look like so far?
Answer: PbS2 + AlPO4 ——> Al2S3 + Pb3(PO4)4
Step 6:
How do I balance the equation?
COMPLETE ANSWER: 3 PbS2 + 4 AlPO4 ——> 2 Al2S3 + Pb3(PO4)4
VIDEO Predicting Chemical Equations Demonstrated Example 5: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Combustion:
C2H6 + O2 ——> ?
Step 1:
Where do I start?
Answer: The products of a combustion equation are always CO2 and H2O?
Step 2:
What does my answer look like so far?
Answer: C2H6 + O2 ——> CO2 + H2O
Step 3:
How do I balance the equation?
COMPLETE ANSWER: 2 C2H6 + 7 O2 ——> 4 CO2 + 6 H2O
VIDEO Predicting Chemical Equations Demonstrated Example 6: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Synthesis:
NH4+ + SO42- ——> ?
Step 1:
Where do I start?
Answer: Bring the two reactant compounds together into a single product compound. In this case bring the two ions together.
Step 2:
What is the compound that contains the two ions?
| NH4+ | SO42- | |
| NH4+ | ||
| Total = | +2 | -2 |
Answer: (NH4)2SO4
Step 3:
What does my answer look like so far?
NH4+ + SO42- ——> (NH4)2SO4
Step 4:
How do I balance the equation?
COMPLETE ANSWER: 2 NH4+ + SO42- ——> (NH4)2SO4
VIDEO Predicting Chemical Equations Demonstrated Example 7: If you have the reactants of a chemical equation and know what type of chemical equation it is then give the products of the chemical equation.
Decomposition:
N2O3 ——> ?
Step 1:
Where do I start?
Answer: Break up the one reactant into many products. It can be two or more. I simply broke the reactant compound into its elements (remember N and O are diatomic).
N2O3 ——> N2 + O2
Step 2:
How do I balance the equation?
COMPLETE ANSWER: 2 N2O3(g) <——> 2 N2(g) + 3 O2(g)
PRACTICE PROBLEMS: Give the balanced complete reaction below.
| Synthesis
Mg + O2 —> ? |
2 Mg + O2 —> 2 MgO |
| Decomposition
AlBr3 —> ? |
2 AlBr3 —> 2 Al + 3 Br2 |
| Combustion
C6H12O6 + O2 —> ? |
C6H12O6 + 6 O2 —> 6 CO2 + 6 H2O |
| Double Replacement
Ca(OH)2 + Na2S —> ? |
Ca(OH)2 + Na2S —> CaS + 2 NaOH |
| Single Replacement
Li + Ba3P2 —> ? |
6 Li + Ba3P2 —> 3 Ba + 2 Li3P |
| Combustion
C4H10 + O2 —> ? |
2 C4H10 + 13 O2 —> 8 CO2 + 10 H2O |
| Double Replacement
MnCO3 + K3PO4 —> ? |
3 MnCO3 + 2 K3PO4 —> Mn3(PO4)2 + 3 K2CO3 |
| Single Replacement
F2 + NH4Br —> ? |
F2 + 2 NH4Br —> 2 NH4F + Br2 |
Chem – Diatomic Molecules in Chemical Equations
What are diatomic molecules?
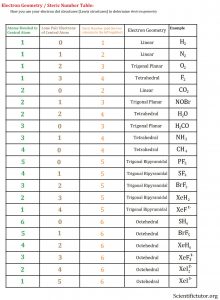
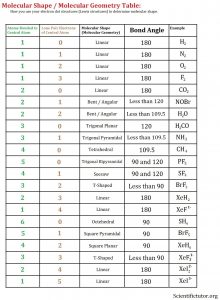
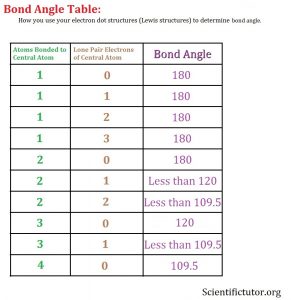
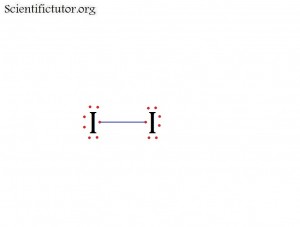
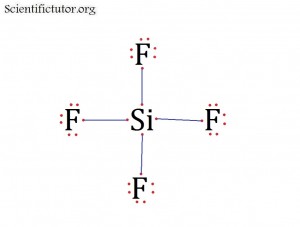
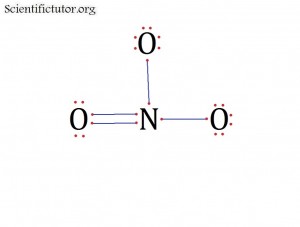
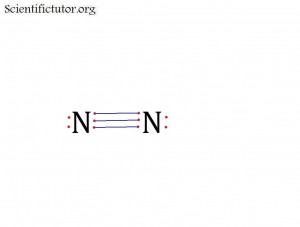
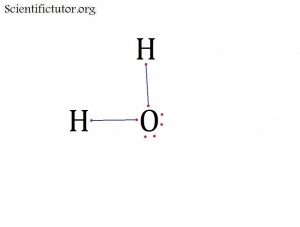
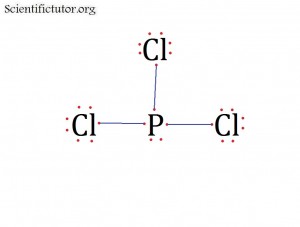
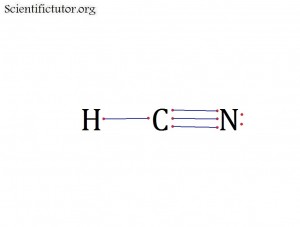
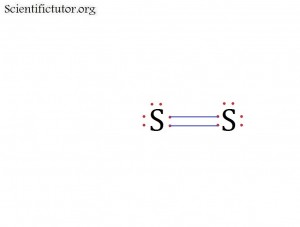
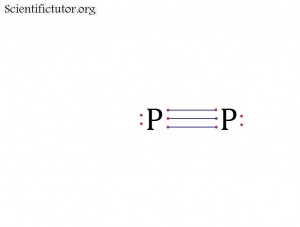
Before we go forward to predicting chemical equations we need to completely explain one concept. That concept is diatomic molecules. First a definition. The word diatomic breaks down into two parts DI- meaning 2 and –ATOMIC meaning atom. So it is molecule made up of two atoms. More specifically it is a molecule made up of two atoms from the same element. These diatomic molecules form because of their abundance in the chemical equation, their stability as a molecule, and their valence electron sharing. You can predict their existence through Valence Electron Dot Structures (Lewis Structures).
The diatomic elements are:
H / N / O / F / Cl / Br / I
They form the diatomic molecules:
H2 / N2 / O2 / F2 / Cl2 / Br2 / I2
How do I memorize these diatomic molecules?
Two ways I have found that help you memorize the diatomic molecules are:
1) Use a mnemonic, a word or words that reminds you of a thing or concept you want to remember. For diatomic molecules people use the mnemonic BrINClHOF. It sounds like Brinkelhoff.
2) Look at the pattern on the periodic table like the picture below.
What is important about diatomic molecules for predicting chemical equations?
When you predict the products of a chemical equation often times it will contain diatomic molecules. What you want to get familiar with in this section is spotting elements that will form those diatomic molecules.
Examples: Identify which elements form diatomic molecules and then write their diatomic molecules. If they do not form diatomic molecules then write NOT.
|
Element |
Diatomic Molecule |
|
Na |
NOT |
|
F |
F2 |
|
S |
NOT |
|
O |
O2 |
PRACTICE PROBLEMS: Identify which elements form diatomic molecules and then write their diatomic molecules. If they do not form diatomic molecules then write NOT.
|
Element |
Diatomic Molecule |
|
Cl |
Cl2 |
|
N |
N2 |
|
P |
NOT |
|
Ba |
NOT |
|
Br |
Br2 |
|
Fe |
NOT |
|
H |
H2 |
Chem – Types of Chemical Equations
What are the different types of chemical equations?
With many chemical equations you can break them down by the type of chemical equation. This helps you to predict how different chemicals will react when mixing them. The different types are below in bold with clear examples. Look at them and see what kind of patterns you can recognize. Use the periodic table or ion periodic table link if you need it.
SYNTHESIS (also known as combination):
Definition: Two or more chemicals on the reactants become a single chemical on the products.
Example 1: Na+(aq) + Cl–(aq) ——> NaCl(aq)
Example 2: Ca(s) + Br2(g) ——> CaBr2(s)
Example 3: 6 C(s) + 6 H2(g) + 3 O2(g) ——> C6H12O6(s)
Generic Example: A + B ——> AB
DECOMPOSITION:
Definition: One chemical on the reactants becoming two or more on the products.
Example 1: LiOH(aq) ——> Li+(aq) + OH–(aq)
Example 2: 2 K2O(s) ——> 4 K(s) + O2(g)
Example 3: 2 HCN(aq) ——> H2(g) + 2 C(s) + N2(g)
Generic Example: AB ——> A + B
SINGLE REPLACEMENT (also known as single displacement):
Definition: One set of chemical partners swap as they go from reactants to products.
Example 1: Na(s) + LiF(aq) ——> Li(s) + NaF(aq)
Example 2: 2 Fe(NO3)3(aq) + 3 Be(s) ——> 3 Be(NO3)2(s) + 2 Fe(s)
Example 3: 3 (NH4)2(CO3)(aq) + 2 PO43-(aq) ——> 2 (NH4)3(PO4)(aq) + 3 CO32-(aq)
Generic Example: AB + C ——> AC + B
DOUBLE REPLACEMENT (also known as double displacement):
Definition: Two sets of chemical partners swap as they go form reactants to products.
Example 1: KBr(aq) + LiCl(aq) ——> KCl(aq) + LiBr(aq)
Example 2: 3 MgS(aq) + 2 Cs3N(aq) ——> 3 Cs2S(aq) + Mg3N2(s)
Example 3: 2 Al(OH)3(aq) + 3 CaSO4(aq) ——> Al2(SO4)3(aq) + 3 Ca(OH)2(aq)
Generic Example: AB + CD ——> AD + CB
COMBUSTION:
Definition: A carbon compound and oxygen combined to become CO2 and H2O
Example 1: CH4(g) + 2 O2(g) ——> CO2(g) + 2 H2O(g)
Example 2: C6H12O6(s) + 6 O2(g) ——> 6 CO2(g) + 6 H2O(g)
Example 3: CH3COOH(aq) + 4 O2(g) ——> 2 CO2(g) + 2 H2O(g)
Generic Example: carbon compound + O2(g) ——> CO2(g) + H2O(g)
Why kinds of questions can they ask you about the types of chemical equations?
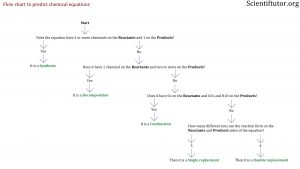
The first kinds of questions you want to be able to answer with the above information is if you are given a complete chemical equation. You want to be able to identify which one of the 5 types of chemical equations it is. To do that the best way is to ask questions about the chemical equations in front of you. Below is a picture of the flow chart of the questions and information that allow you to either confirm or deny the different types of chemical equations. Look to the demonstrated example videos and text to understand how to use the flow chart.
VIDEO Types of Chemical Equations Demonstrated Example 1:Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
Rb2S(s) ——> 2 Rb+(aq) + S2-(aq)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If Yes: Then it is a DECOMPOSTION reaction
VIDEO Types of Chemical Equations Demonstrated Example 2: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
2 Ag3P(s) + 3 V(s) ——> V3P2(s) + 6 Ag(s)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If No: Then it is NOT a combustion
Step 4:
Answer: Follow the NO arrows on the flow chart and ask the next question.
How many different ions can the reaction form on the reactants and products sides of the equation?
Ag forms a positive ion (that is 1), P forms a negative ion (that is 2), V forms a positive ion (that is 3)
If 3 on each side: Then it is a SINGLE REPLACEMENT
VIDEO Types of Chemical Equations Demonstrated Example 3: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
C3H8(l) + 10 O2(g) ——> 3 CO2(g) + 4 H2O(g)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If Yes: Then it is a combustion reaction
VIDEO Types of Chemical Equations Demonstrated Example 4: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
2 NaNO3(aq) + Mg(C2H3O2)2(aq) ——> Mg(NO3)2(aq) + 2 NaC2H3O2(aq)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If No: Then it is NOT a combustion
Step 4:
Answer: Follow the NO arrows on the flow chart and ask the next question.
How many different ions can the reaction form on the reactants and products sides of the equation?
Na forms a positive ion (that is 1), NO3 forms a negative ion (that is 2), Mg forms a positive ion (that is 3), C2H3O2 forms a negative ion (that is 4)
If 4 on each side: Then it is a double replacement
VIDEO Types of Chemical Equations Demonstrated Example 5: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
3 Be(s) + N2(g) ——> Be3N2(s)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If Yes: Then it is a synthesis reaction
PRACTICE PROBLEMS: Determine which type of chemical equation each of them is below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also. Try to answer these questions WITHOUT the flow chart but use it if needed.
| H2SO4(aq) —–> H2(g) + S(s) + 2 O2(g) |
| Answer: Decomposition |
| Ba(OH)2(aq) + 2 HCl(aq) ——> 2 H2O(l) + BaCl2(aq) |
| Answer: Double Replacement |
| N2(g) + 3 H2(g) —-> 2 NH3(g) |
| Answer: Synthesis |
| 6 Ag(s) + Ca3(PO4)2(s) ——> 3 Ca(s) + 2 Ag3PO4(s) |
| Answer: Single Replacement |
| 3 NiF2(s) + 2 (NH4)3P(s) ——> 6 NH4F(aq) + Ni3P2(s) |
| Answer: Double Replacement |
| 2 C4H10(l) + 13 O2(g) ——> 8 CO2(g) + 10 H2O (g) |
| Answer: Combustion |
| 3 Na(s) + CrCl3(s) ——> 3 NaCl(aq) + Cr(s) |
| Answer: Single Replacement |
| 2 MnI3(s) ——> 3 I2(g) + Mn(s) |
| Answer: Decomposition |
Chem – Tips and Tricks for Balancing Chemical Equations
What are some of the tricks for balancing a chemical equation?
For the simple chemical equations in the previous section, the process is pretty straightforward, but there are some chemicals equations that many people have trouble balancing. Those chemical equations usually include situations where you need to keep balancing until you get to high coefficient numbers or when you have lots of elements on both sides of the chemical equations. In this section I will show you some tricks to make the these kinds of chemical equations much easier.
VIDEO Balancing Chemical Equation Demonstrated Example 3: Balance the chemical equation below.
Al(OH)3(aq) + Li2CO3(aq) ——> LiOH(aq) + Al2(CO3)3(s)
Step 1:
How do we start to balance a chemical equation?
Answer: Same as before, first we write down the chemical equations on our paper and then we draw a line down the yield sign to separate the two halves of a chemical equation.
| Al(OH)3(aq) + Li2CO3(aq) — | —> LiOH(aq) + Al2(CO3)3(s) |
| Al = 1 | Al = 2 |
| OH = 3 | OH = 1 |
| Li = 2 | Li = 1 |
| CO3 = 1 | CO3 = 3 |
Step 2:
Then we count up all the elements on each side of the equation and assume that all the coefficients are 1. However, in this case we have polyatimic ions. If the polyatomic ions appear the same on both sides of the chemical equations, we can count the polyatomic ions as one whole instead of individual elements. This makes counting much simpler.
| Al(OH)3(aq) + Li2CO3(aq) — | —> LiOH(aq) + Al2(CO3)3(s) |
| Al = 1 | Al = 2 |
| OH = 3 | OH = 1 |
| Li = 2 | Li = 1 |
| CO3 = 1 | CO3 = 3 |
Step 3:
Now start balancing the equation by adding coefficients. I will start with the Aluminum on the left side.
| 2 Al(OH)3(aq) + Li2CO3(aq) — | —> LiOH(aq) + Al2(CO3)3(s) |
| Al = 2 | Al = 2 |
| OH = 6 | OH = 1 |
| Li = 2 | Li = 1 |
| CO3 = 1 | CO3 = 3 |
Step 4:
Next I try the CO3 on the left side.
| 2 Al(OH)3(aq) + 3 Li2CO3(aq) — | —> LiOH(aq) + Al2(CO3)3(s) |
| Al = 2 | Al = 2 |
| OH =6 | OH = 1 |
| Li = 6 | Li = 1 |
| CO3 = 3 | CO3 = 3 |
Step 5:
Then I Li and OH on the right side.
| 2 Al(OH)3(aq) + 3 Li2CO3(aq) — | —> 6 LiOH(aq) + Al2(CO3)3(s) |
| Al = 2 | Al = 2 |
| OH =6 | OH = 6 |
| Li = 6 | Li = 6 |
| CO3 = 3 | CO3 = 3 |
Step 6:
All the elements and polyatomic ions are the same on both sides so I am done.
COMPLETE ANSWER: 2 Al(OH)3(aq) + 3 Li2CO3(aq) ——> 6 LiOH(aq) + Al2(CO3)3(s)
VIDEO Balancing Chemical Equation Demonstrated Example 4: Balance the chemical equation below.
C5H7OH(l) + O2(g) ——> CO2(g) + H2O(g)
Step 1:
How do we start to balance a chemical equation?
Answer: Same as before, first we write down the chemical equations on our paper and then we draw a line down the yield sign to separate the two halves of a chemical equation.
| C5H7OH(l) + O2(g) — | —> CO2(g) + H2O(g) |
| C = 5 | C = 1 |
| H = 8 | H = 2 |
| O = 3 | O = 3 |
Step 2:
Then we count up all the elements on each side of the equation and assume that all the coefficients are 1.
| C5H7OH(l) + O2(g) — | —> CO2(g) + H2O(g) |
| C = 5 | C = 1 |
| H = 8 | H = 2 |
| O = 3 | O = 3 |
Step 3:
Start to try to balance them with coefficients we are going to start with 5 in front of the CO2 on the right side. It is best to balance the carbons and hydrogen first in these kinds of reactions.
| C5H7OH(l) + O2(g) — | —> 5 CO2(g) + H2O(g) |
| C = 5 | C = 5 |
| H = 8 | H = 2 |
| O = 3 | O = 11 |
Step 4:
Then I would change the hydrogen on the right by putting a 4 in front of H2O.
| C5H7OH(l) + O2(g) — | —> 5 CO2(g) + 4 H2O(g) |
| C = 5 | C = 5 |
| H = 8 | H = 8 |
| O = 3 | O = 14 |
Step 5:
Now we run into a problem. The oxygens are an even number on the right side and an odd number on the left side. To simplify my problem all we have to do is make the oxygens an even number on the left side (reactants). How do I do that? Change the coefficient of C5H7OH to 2.
| 2 C5H7OH(l) + O2(g) — | —> 5 CO2(g) + 4 H2O(g) |
| C = 10 | C = 5 |
| H = 16 | H = 8 |
| O = 4 | O = 14 |
Step 6:
This unbalances my right side again so I go back to that side and fix it. Change coefficient in front of CO2 to 10.
| 2 C5H7OH(l) + O2(g) — | —> 10 CO2(g) + 4 H2O(g) |
| C = 10 | C = 10 |
| H = 16 | H = 8 |
| O = 4 | O = 24 |
Step 7:
Lets fix the hydrogen on the right by changing the coefficient of the H2O to 8.
| 2 C5H7OH(l) + O2(g) — | —> 10 CO2(g) + 8 H2O(g) |
| C = 10 | C = 10 |
| H = 16 | H = 16 |
| O = 4 | O = 28 |
Step 8:
Now we have to make the oxygen on the left side match the right. Remember that 2 oxygen are already contained in the first compound on the right side. So we can find out what the coefficient of O2 by the oxygen on the right minus 2 divided by 2 = 28 – 2 / 2 = 13
| 2 C5H7OH(l) + 13 O2(g) — | —> 10 CO2(g) + 8 H2O(g) |
| C = 10 | C = 10 |
| H = 16 | H = 16 |
| O = 28 | O = 28 |
Step 9:
All the elements are the same on both sides so I am done.
COMPLETE ANSWER: 2 C5H7OH(l) + 13 O2(g) ——> 10 CO2(g) + 8 H2O(g)
PRACTICE PROBLEMS: Balance the chemical equations below.
| Na3PO4(aq) + CaCl2(aq) ——> Ca3(PO4)2(s) + NaCl(aq) |
| Answer: 2 Na3PO4(aq) + 3 CaCl2(aq) ——> Ca3(PO4)2(s) + 6 NaCl(aq) |
| K2(SO3)(aq) + Mg3(PO4)2(s) ——> MgSO3(aq) + K3PO4(aq) |
| Answer: 3 K2(SO3)(aq) + Mg3(PO4)2(s) ——> 3 MgSO3(aq) + 2 K3PO4(aq) |
| C2H6(l) + O2(g) ——> CO2(g) + H2O(g) |
| Answer: 2 C2H6(l) + 7 O2(g) ——> 4 CO2(g) + 6 H2O(g) |
| C6H11OH(l) + O2(g) ——> CO2(g) + H2O(g) |
| Answer: 2 C6H11OH(l) + 17 O2(g) ——> 12 CO2(g) + 12 H2O(g) |
Chem – Balancing Chemical Equations
How do you balance a chemical equation?
The next step is to balance entire chemical equations. Balancing chemical equations comes from the concept that the amount of stuff you put into the reactants side of the chemical equation should be the same amount of stuff you get out of the products side of the chemical equation. Another way that chemistry books and teachers usually say it is that mass is not gained or lost in a chemical equation. It is like baking cookies. If you double the ingredients you mix together then you get double the cookies in the end.
Remember to always total up all the elements on both sides of the chemical equation and any chemical compound not showing a number in front of it has a coefficient of 1. Don’t try to total them in your head. Write them down on a piece of paper or drawing program on your computer. It takes approximately 15 attempts at balancing chemical equations until you get comfortable with it. It takes approximately 30 attempts until you are good at it. It takes approximately 60 attempts until you can actually start do these in your head. If you do not write down the first 60 attempts on paper you will never be able to do them in your head. Trust me I have done thousands of them. The strategy is to take it slow and steady with balancing. Don’t get frustrated; just keep trying to move forward.
Examples: Balance the following chemical equations.
| ___N2(g) + ___O2(g) —-> ___N2O3(g) |
| 2 N2(g) + 3 O2(g) —-> 2 N2O3(g) |
| 1 |
| ___CaCl2(aq) + ___Al2(SO3)3(aq) —-> ___CaSO3(aq) + ___AlCl3(aq) |
| 3 CaCl2(aq) + Al2(SO3)3(aq) —-> 3 CaSO3(aq) + 2 AlCl3(aq) |
VIDEO Balancing Chemical Equation Demonstrated Example 1: Balance the unbalanced chemical equation below.
MgBr2(aq) + NaI(aq) ——> MgI2(aq) + NaBr(aq)
Step 1:
How do we start to balance a chemical equation?
Answer: First we write down the chemical equations on our paper and then we draw a line down the yield sign to separate the two halves of a chemical equation.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + NaBr(aq) |
| Mg = | Mg = |
| Br = | Br = |
| Na = | Na = |
| I = | I = |
Step 2:
What do we do next?
Answer: Below the reactants and products side we list each element followed by an equal sign. Make sure you write both sides in the same element order to save you head ache later.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + NaBr(aq) |
| Mg = | Mg = |
| Br = | Br = |
| Na = | Na = |
| I = | I = |
Step 3:
Now count up all the elements on each side of the equation and assume that all the coefficients are 1.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + NaBr(aq) |
| Mg = 1 | Mg = 1 |
| Br = 2 | Br = 1 |
| Na = 1 | Na = 1 |
| I = 1 | I = 2 |
Step 4:
What we want to focus on from here on out is the differences in the numbers between the reactants and products. What differences do you notice in this problem?
Answer: Br and I.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + NaBr(aq) |
| Mg = 1 | Mg = 1 |
| Br = 2 | Br = 1 |
| Na = 1 | Na = 1 |
| I = 1 | I = 2 |
Step 5:
We can only change these differences by using coefficients to change the chemical equation. We CANNOT EVER change the subscripts. You can start by changing any coefficient you like. It does not really matter where you start. I will begin by changing the coefficient of NaBr to a 2.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + 2 NaBr(aq) |
| Mg = 1 | Mg = 1 |
| Br = 2 | Br = 1 |
| Na = 1 | Na = 1 |
| I = 1 | I = 2 |
Step 6:
Immediately after that you want to change the number of each element below the side you changed. The 2 NaBr changes the Br but it also affects the Na.
| MgBr2(aq) + NaI(aq) — | —> MgI2(aq) + 2 NaBr(aq) |
| Mg = 1 | Mg = 1 |
| Br = 2 | Br = 2 |
| Na = 1 | Na = 2 |
| I = 1 | I = 2 |
Step 7:
Now where is there a difference?
Answer: The Na and I. So I will put a 2 coefficient in front of NaI.
| MgBr2(aq) + 2 NaI(aq) — | —> MgI2(aq) + 2 NaBr(aq) |
| Mg = 1 | Mg = 1 |
| Br = 2 | Br = 2 |
| Na = 2 | Na = 2 |
| I = 2 | I = 2 |
Step 8:
As we check for any more differences we find none. Therefore, we are done balancing the equation.
COMPLETE ANSWER: MgBr2(aq) + 2 NaI(aq) ——> MgI2(aq) + 2 NaBr(aq)
VIDEO Balancing Chemical Equation Demonstrated Example 2:Please balance the unbalanced chemical equation below.
N2(g) + H2(g) —-> NH3(g)
Step 1:
How do we start to balance a chemical equation?
Answer: First we write down the chemical equations on our paper and then we draw a line down the yield sign to separate the two halves of a chemical equation.
| N2(g) + H2(g) — | –> NH3(g) |
| N = | N = |
| H = | H = |
Step 2:
What do we do next?
Answer: Below the reactants and products side we list each element followed by an equal sign. Make sure you write both sides in the same element order to save you head ache later.
| N2(g) + H2(g) — | –> NH3(g) |
| N = | N = |
| H = | H = |
Step 3:
Now count up all the elements on each side of the equation and assume that all the coefficients are 1.
| N2(g) + H2(g) — | –> NH3(g) |
| N = 2 | N = 1 |
| H = 2 | H = 3 |
Step 4:
What we want to focus on from here on out is the differences in the numbers between the reactants and products. What differences do you notice in this problem?
Answer: N and H.
| N2(g) + H2(g) — | –> NH3(g) |
| N = 2 | N = 1 |
| H = 2 | H = 3 |
Step 5:
We can only change these differences by using coefficients to change the chemical equation. We CANNOT EVER change the subscripts. You can start by changing any coefficient you like. It does not really matter where you start. I will begin by changing the coefficient of NH3 to a 2.
| N2(g) + H2(g) — | –> 2 NH3(g) |
| N = 2 | N = 1 |
| H = 2 | H = 3 |
Step 6:
Immediately after that you want to change the number of each element below the side you changed. The 2 NH3 changes the N but it also affects the H.
| N2(g) + H2(g) — | –> 2 NH3(g) |
| N = 2 | N = 2 |
| H = 2 | H = 6 |
Step 7:
Now where is there a difference?
Answer: The H. So I will put a 3 coefficient in front of the H2.
| N2(g) + 3 H2(g) — | –> 2 NH3(g) |
| N = 2 | N = 2 |
| H = 6 | H = 6 |
Step 8:
As we check for any more differences we find none. Therefore, we are done balancing the equation.
COMPLETE ANSWER: N2(g) + 3 H2(g) —-> 2 NH3(g)
PRACTICE PROBLEMS: Balance the chemical equations below.
| H2O(l) —> H2(g) + O2(g) |
| Answer: 2 H2O(l) —> 2 H2(g) + O2(g) |
| H2SO4(aq) —> H2(g) + S(s) + O2(g) |
| Answer: H2SO4(aq) —> H2(g) + S(s) + 2 O2(g) |
| OF2(g) + NH3(g) —> N2F4(g) + O2(g) + H2(g) |
| Answer: 2 OF2(g) + 2 NH3(g) —> N2F4(g) + O2(g) + 3 H2(g) |
| Fe3+(aq) + CO32-(aq) —-> Fe2(CO3)3(s) |
| Answer: 2 Fe3+(aq) + 3 CO32-(aq) —> Fe2(CO3)3(s) |
| Ba(OH)2(aq) + HCl(aq) —-> H2O(l) + BaCl2(aq) |
| Answer: Ba(OH)2(aq) + 2 HCl(aq) —> 2 H2O(l) + BaCl2(aq) |
Chem – Coefficients
What are chemical equation coefficients?
Chemical equations also need to be balanced. The above chemical equations are not balanced. All further chemical equations on this page should be balanced (If they are not practice problems). This means they have to have the same amount of each element on the reactants side as they do on the products side. The trick to balancing is that you cannot change the compounds by changing the subscript. However, you can put a number in the front of them that is called the coefficient. In math terms, this coefficient multiplies all the elements of a single compound behind it. Before we start balancing equations let us try practicing using coefficients.
Examples: Label how many of each element is contained in the coefficient and compound combination.
| 2 NaCl | 2 Na and 2 Cl |
| 6 P4S3 | 24 P and 18 S |
| 3 Mg3(PO4)2 | 9 Mg, 6 P, and 24 O |
VIDEO Coefficient Demonstrated Example 1: Label how many of each element is contained in the coefficient and compound combination.
4 CaBr2
Step 1:
What is the subscript of Ca?
Answer: 1
Step 2:
What is the coefficient of the compound?
Answer: 4
Step 3:
What is the coefficient multiplied by the subscript?
Answer: 4 * 1 = 4 So there are 4 Ca
Step 4:
What is the subscript of Br?
Answer: 2
Step 5:
What is the coefficient of the compound?
Answer: 4
Step 6:
What is the coefficient multiplied by the subscript?
Answer: 4 * 2 = 8 So there are 8 Br
Step 7:
What is the total of all the elements in the compound with the coefficient?
COMPLETE ANSWER: 4 Ca and 8 Br
VIDEO Coefficient Demonstrated Example 2:Label how many of each element is contained in the coefficient and compound combination.
6 Al2(CO3)3
Step 1:
What is the subscript of Al?
Answer: 2
Step 2:
What is the coefficient?
Answer: 6
Step 3:
What is the coefficient multiplied by the subscript of Al?
Answer: 6 * 2 = 12 So there are 12 Al
Step 4:
What is the subscript inside the parenthesis of C?
Answer: 1
Step 5:
What is the coefficient?
Answer: 6
Step 6:
What is the subscript outside the parenthesis?
Answer: 3
Step 7:
What is the subscript inside the parenthesis of C multiplied by the coefficient multiplied by the subscript outside the parenthesis?
Answer: 1 * 6 * 3 = 18 So there are 18 C
Step 8:
What is the subscript inside the parenthesis of O?
Answer: 3
Step 9:
What is the coefficient?
Answer: 6
Step 10:
What is the subscript outside the parenthesis?
Answer: 3
Step 11:
What is the subscript inside the parenthesis of O multiplied by the coefficient multiplied by the subscript outside the parenthesis?
Answer: 3 * 6 * 3 = 54 So there are 54 O
Step 12:
What is the total of all the elements in the compound with the coefficient?
COMPLETE ANSWER: 12 Al, 18 C, and 54 O
PRACTICE PROBLEMS: Label how many of each element is contained in the coefficient and compound combination.
| 5 Na2S | 10 Na and 5 S |
| 2 Ni3P | 6 Ni and 2 P |
| 4 Sr3(PO3)2 | 12 Sr, 8P, and 24 O |
| 3 SiH4 | 3 Si and 12 H |
| 1 Se5Br6 | 5 Se and 6 Br |
| 6 Be(NO3)2 | 6 Be, 12 N, and 36 O |
Chem – States of Matter in a Chemical Equation
How are states of matter displayed in a chemical equation?
In this section we are going to show you how states of matter are displayed in a chemical equation. The states of matter are gas, like the air we breath, liquid, like the water we drink, solid, like the ground we are standing on, and aqueous like the sugary drink we taste.
The notation for each state is as follows below:
Gas = (g)
Liquid = (l)
Solid = (s)
Aqueous = (aq)
All the state of matter notations go at the end of each chemical next to the subscript.
Examples:
H2SO4(aq) —> H2(aq) + S(s) + O2(aq)
H2(g) + O2(g) —-> H2O(l)
In the first equation, H2SO4 is in an aqueous state. In the second equation, H2O is in a liquid state.
Use the practice problems below to bring together your understanding of reactants versus products and the different states of matter.
PRACTICE PROBLEMS: Give the states of matter for the requested chemicals below.
What are the states of matter for the products of the chemical equation below?
Ag(s) + Ca3(PO4)2(s) —-> Ca(aq) + Ag3PO4(s)
Answer: Ca = aqueous, Ag3PO4 = solid
What are the states of matter for the reactants of the chemical equation below?
C4H10 (l) + O2 (g) —-> CO2 (g) + H2O(g)
Answer: C4H10 = liquid, O2 = gas
What are the states of matter for the products of the chemical equation below?
Ba(OH)2(aq) + HCl(aq) —-> H2O(l) + BaCl2(aq)
Answer: H2O = liquid, BaCl2 = aqueous
What are the states of matter for the reactants of the chemical equation below?
C4(s) + O2(g) —> CO2(g)
Answer: C4 = solid, O2 = gas
Chem – Definition and Layout of a Chemical Equation
How are chemical equations organized?
Chemical equations are a series of elements or compounds that when added together under the right conditions cause a chemical reaction to happen and produce a new arrangement of elements or compounds. Those elements do not change, but their bonds to other elements can.
Examples:
H2 + O2 —-> H2O
NaCl + MgBr2 —-> NaBr + MgCl2
The chemical equations also break down into a few different parts that are labeled. On the left side of the arrow sign are the reactants. On the right side of the arrow sign are the products. The arrow sign is called the yield. THE REACTANTS ARE ALWAYS ON THE LEFT SIDE UNLESS YOU ARE TOLD OTHERWISE! THE PRODUCTS ARE ALWAYS ON THE RIGHT SIDE UNLESS YOU ARE TOLD OTHERWISE!
Example:
| Reactants | Yield | Products |
| C6H12O6 + O2 | —-> | CO2 + H2O |
Chem – LESSON 10: Chemical Equations
What is the lesson about?
This lesson is about how different substances can interact to form new ones. How you turn the gasoline in your car into carbon dioxide. How plants turn soil particles and sunlight into new leaves. The ingredients needed to make kitchen or farming tools. All things that people make at some point go through a chemical reaction to acquire the properties that we want them to have.
Why is it critical to understand?
In terms of learning chemistry, this starts to weave together many of the separate branches you have been learning. All learning before and all learning after, this is really just built for the understanding of chemical equations. You could say that the entire reason chemistry was made into a science was because of the foundations of this chapter. How we make new products has always been important to humans and the chemical reactions are the heart of that. This is why you are supposed to follow a cooking recipe as it appears. A recipe is nothing more than a chemical equation and if you do not follow it you will get dish that usually will not taste quite right.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to sections on Ions, Covalent Ionic and Metallic Bonds, Introduction to Polyatomic Ions, Identifying Polyatomic Ions in Compounds, Forming Ionic Compounds, and Breaking Apart Ionic Compounds.
New Learning Sections:
—> Definition and Layout of a Chemical Equation
—> States of Matter in a Chemical Equation
—> Coefficients
—> Balancing Chemical Equations Part 1
—> Tips and Tricks for Balancing Equations Part 2
—> Types of Chemical Equations
—> Diatomic Molecules in Chemical Equations
—> Prediction types of Chemical Equations Part 1
—> College: Prediction types of Chemical Equations Part 2
Reference Pages:
—> Types of Chemical Equations Flow Chart
—> List of Diatomic Molecules
Worksheets:
—> Chemical Equations Worksheet 1
—> Chemical Equations Worksheet 1 WITH ANSWERS
Chem – College: Different Types of Solids
What are the different types of solids?
VIDEO explanation of the Different Types of Solids.
Depending on the book and teacher you have they might describe the names of the different types of solids in a slightly different way. Keep in mind the names I describe here are the most common ones I have heard. The table below has the different types of solids.
| Types | Examples |
| Metallic | A cooking pan |
| Ionic | Salt |
| Molecular | Ice or Dry Ice |
| Network Covalent | Diamond |
| Amorphous | Plastic |
The first 4 types of solids (metallic, ionic, molecular, and Network Covalent) are all referred to as crystals because they have a very organized and repeating structure. The last type, amorphous is not a crystal because it does not have a repeating chemical structure. Mostly what confuses people on this section of chemistry is determining which types of solid a particular chemical is. Lets look at an example below:
Example: NH3 will form what type of solid?
Answer: Molecular
HOW THE HECK DID YOU GET THAT ANSWER? Good question. It goes back to a lot a material we have learn previously but especially focusing on the sections of covalent and ionic bonds and the Lewis Structures lesson up to completing the octet. I GUARANTEE YOU IF YOU DO NOT UNDERSTAND THESE PREVIOUS SECTIONS YOU WILL NOT UNDERSTAND ANYTHING I AM ABOUT TO WRITE BELOW.
The question to keep in mind during this section is what are the weakest possible bonds that form in each chemical compound? If you can identify those bonds or forces then you will be able to answer the practice problems below and the questions you will have on your test. Below is another table about how I want you to think as you are going through the problems.
| Types | Weakest Bonds |
| Metallic | Metallic |
| Ionic | Ionic |
| Molecular | Hydrogen, Dipole-Dipole, or London-Dispersion |
| Network Covalent | Covalent |
| Amorphous | Any but mostly covalent |
Metallic: Any chemical that contains just metals will contain only metallic bonds. Examples: Al2Mn3, Na2Zn, Cu2Pb
Ionic: Any compound that contains a least one metal and at least one non-metal contains only ionic bonds. Examples: KBr, FeCl3 , MgF2
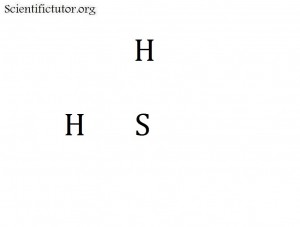
Molecular: Will only contain non-metal elements and therefore have covalent bonds but these are not the only types of bonds they contain. Their weakest bonds will be Hydrogen, Dipole-Dipole, or London-Dispersion. Under extreme cold temperatures and high pressures these can form solids. How is it best to distinguish these from Network Covalent solids? Molecular solids can be drawn as a Lewis Structure but Network Covalent cannot. Examples: NH3, H2S, CO2 (dry ice)
Network Covalent: Will have only non-metals and therefore contain covalent bonds. Unlike the molecular you will not be able to draw these as a Lewis Structure (only exception is SiO2). Examples: C4 (diamond), S8, PN3
Amorphous: This category could include anything because it is not dependent on what elements are in it. Amorphous (without form) is only dependent on how the elements are arranged relative to each other. They can have no repeating pattern only a disorganized mix. Examples: Soil (dirt), rubber, plastic
PRACTICE PROBLEMS: Name the type of solid for each chemical below. Your options are metallic, ionic, molecular, or covalent network. Amorphous will not be used in these practice problems.
| PdBr2 | Ionic |
| H2O | Molecular |
| FeZn | Metallic |
| BC2 | Network Covalent |
| CaI | Ionic |
| SO2 | Molecular |
| SiH4 | Molecular |
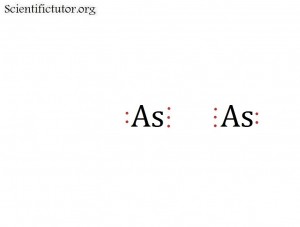
| ClAs | Network Covalent |
| Ni3Al2 | Metallic |
| F2 | Molecular |
Chem – College: Molecular Forces and States of Matter
How do molecular forces affect states of matter?
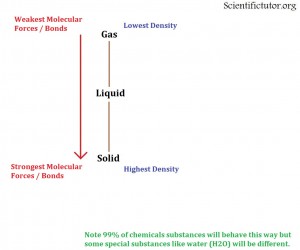
When you are comparing two chemicals with very similar molar mass and you are trying to determine any possible difference in state between them then you have to move on to the types of molecular forces holding them together. We can recall our previous section on covalent and ionic bonds explained INTRAMOLECULAR forces or bonds and our previous section on Intermolecular forces from Lewis Structures explained INTERMOLECULAR forces or bonds. It turns out that you can use these forces to make predictions as to what the density or what the state of matter is. In general the STRONGER the forces between atoms or molecules the MORE DENSE the chemical and the MORE LIKELY it is to be a SOLID. The WEAKER the forces between two atoms or molecules the LESS DENSE the chemical and the MORE LIKELY it is to be a GAS. This relationship is displayed in the picture I have created below.
Now that we know the relationship between the molecular forces and the states of matter we also have to be told about which molecular forces are stronger than others. The strength of these molecular forces or bonds is in order below:
| Forces/Bonds | Type | |
| Strongest | Covalent | INTRAMOLECULAR |
| Metallic | INTRAMOLECULAR | |
| Ionic | INTRAMOLECULAR | |
| Hydrogen | INTERMOLECULAR | |
| Dipole-Dipole | INTERMOLECULAR | |
| Weakest | London Dispersion | INTERMOLECULAR |
As far as INTRAMOLECULAR forces. If you only know that a substance has either covalent bonds, ionic bonds, or metallic bonds and you know nothing else about it you can make a prediction that the unknown substance will be very dense and will most likely be a solid in the conditions at the surface of the Earth.
Examples: Answer the questions below using the intramolecular and intermolecular forces table above.
VIDEO Molecular Forces and States of Matter Explanation for Example 1
A reaction with an unknown substance allowed us to find that the unknown substance had only covalent bonds. Is the unknown substance more likely to be a liquid or is it more likely to be a solid?
Answer: solid
VIDEO Molecular Forces and States of Matter Explanation for Example 2
A large amount of the two chemicals H2S and O2 are forced in to the same chamber. We see one existing in the gas state and one in the liquid state with no chemical reaction between them. What state is each chemical in and give your reasoning as to why?
Answer: H2S is in the liquid state because it has Dipole-Dipole forces. O2 is the gas state because it has London-Dispersion forces.
VIDEO Molecular Forces and States of Matter Explanation for Example 3
C4 and LiF are each tested to see how dense they are. Before the test take place predict which one will be less dense.
Answer: Because C4 has covalent forces and LiF has ionic forces, LiF is less dense.
VIDEO Molecular Forces and States of Matter Explanation for Example 4
What can we predict about the chemical forces in Earth’s atmosphere?
Answer: Earth’s atmosphere is in a gas state. Therefore, it is most likely held together by London Dispersion forces.
PRACTICE PROBLEMS: Answer the questions below using the intramolecular and intermolecular forces table above.
If you have the two chemicals of NaCl and NHF2 in the same container and one appears to be a gas and the other a liquid? Which one is the gas?
Answer: NHF2 will be the liquid because it has hydrogen bonding and those bonds are weaker than the ionic bonding of NaCl.
SiH4 and PH3 are placed in the same container. When I look at the container it appears one of the chemicals is liquid and one of the chemicals is solid. Which one is most likely to be the liquid and why?
Answer: SiH4 is most likely to be the liquid because it has weaker forces (London-Dispersion).
If you have the two solid chemicals of H2O and CH4 in the same container and you raise the temperature, which one will melt first and why?
Answer: The CH4 because it has London-Dispersion forces which are weaker than the hydrogen bonding of H2O.
A group of astronomers discover a new planet and by looking at it they can tell that the planet is composed of mainly three chemical compounds, Al3Fe2, Br2 and SeCl2. If there is life on this planet what chemical would it breath, what chemical would it swim in, and what chemical would it stand on? Explain your reasoning.
Answer: It would breathe Br2 because it is held together by London-Dispersion forces. It would swim in SeCl2 because it is held together by Dipole-Dipole forces. It would stand on Al3Fe2 because it is held together by metallic bonds.
Chem – College: Molar Mass and States of Matter
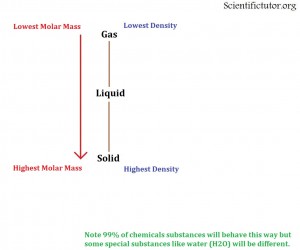
How does molar mass affect the state of matter?
The molar mass of an element or compound has a lot to do with determining its possible state of matter (solid, liquid, gas) generally as the molar mass of a substance increases then it gets more and more dense. As the density increases it is more likely to be in the solid state. Therefore;
As the molar mass of a chemical INCREASES it is more likely to be the MOST dense state possible (usually SOLID).
As the molar mass of a chemical DECREASES it is more likely to be the LEAST dense state possible (usually GAS).
Examples: Answer the questions below. Molar mass versus states of matter examples below.
From the compounds below which one is most likely to be a solid?
NaCl, LiF, RbI, KBr
Answer: RbI
Out of the molecules below which one is most likely to be a gas?
F2, Cl2, Br2, I2
Answer: F2
PRACTICE PROBLEMS: Answer the questions below.
From the compounds below which one is most likely to be a gas?
H2S, H2O, H2Se, H2Te
Answer: H2O
Out of the molecules below which one is most likely to be a Solid?
Ar, Xe, Ne, He
Answer: Xe
If you have a 4 separate containers of each chemical below and 3 of them are solids and one is a liquid, which one is most likely to be the liquid?
CsI, RbBr, LiF, KCl
Answer: LiF
Which chemical below will become a gas first?
NH3, PF3, AsBr3, PI3
Answer: NH3
Chem – Phase Diagrams
What are phase diagrams?
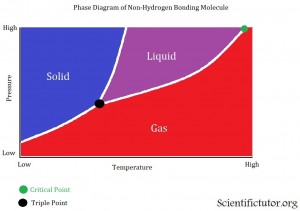
Phase diagrams are a way to display how the conditions of pressure and temperature act separately or together to change or create the different phases of solid, liquid, and gas. Now that we know some of the terminology used with phase diagrams from the last section we can move on to displaying and analyzing them. All phase diagrams display the same information but depending on which chemical you are analyzing in your phase diagrams it can look slightly different. EACH PHASE DAIGRAMS ONLY DISPLAYS ONE CHEMICAL AT A TIME. Lets us take a look at the first and more popular type of phase diagram. The example picture is below.
VIDEO explanation of Phase Diagram 1
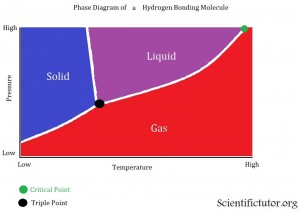
The VERTICAL AXIS (up and down) of the phase diagram above is PRESSURE. As we go further up we increase the pressure. The HORIZONTAL AXIS (left and right) of the phase diagrams above is TEMPERATURE. As we go further to the right we increase the temperature. The different lines displayed inside the graph are the barriers between the different phases. The different colored sections are displaying where this chemical would be a solid, liquid, or gas. Gases on phase diagrams are sometimes called vapor. Last there are two different points that are usually displayed on the phase diagram. The first (about in the middle of the diagrams) is the triple point, where are three phases of a chemical can exist at the same time. This means if you had a container that existed at the exact conditions (of pressure and temperature) as their triple point and you looked into the container you would see 1/3 of it as a solid, 1/3 as a liquid, and 1/3 as a gas. The second point (at the top right) on the phase diagrams is the critical point. Sometimes called the critical temperature point. The critical point is when a certain temperature is reached where the chemical can no longer go back to either the liquid or the solid phase. No matter how much pressure you apply on that chemical it will remain a gas as long as the critical temperature is reached. That last thing to add is this phase diagram is of a substance that does not have hydrogen bonding, which we learned about in the Lewis Structures lesson. Therefore, the barrier (line) between the solid and liquid phase is bent toward the right. This indicates that at high pressures the liquid phase will disappear and only the solid or gas phase will be left. It is something that is not directly displayed in this graph but it is suggested from the information given.
VIDEO explanation of Phase Diagram 2
All the points and definitions of a phase diagram are the same as in the first explanations. The only difference with this one is that it is of a chemical (H2O) that has hydrogen bonding. Therefore, the barrier (line) between the solid and liquid phase is bent toward the left. This indicates that at high pressures the solid phase will disappear and only the liquid or gas phase will be left.
Examples: Answer the questions below the phase diagram pictures. Each axis of the graph only has numbers and no units. You will learn about units of pressure and temperature in the gas laws lesson later. These phase diagrams are created only for the sake of problems you might see in a class room and are not taken from real measurements or experiments so don’t use them to perform dangerous testing of the chemicals displayed. Phase diagram examples below.
(DO EXAMPLES WITH PICTURES)
PRACTICE PROBLEMS: Answer the questions below the phase diagram pictures. Each axis of the graph only has numbers and no units. You will learn about units of pressure and temperature in the gas laws lesson later. These phase diagrams are created only for the sake of problems you might see in a class room and are not taken from real measurements or experiments so don’t use them to perform dangerous testing of the chemicals displayed.
(DO EXAMPLES WITH PICTURES)
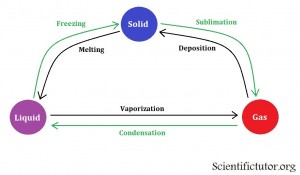
Chem – Definitions of Transition Between States of Matter
How do we cause things to go between different states of matter?
The previous two sections in this lesson suggested how you could change conditions like temperature and pressure to achieve the state of matter that you want from a chemical. However, this section will complete that thought and show you how it is commonly displaced in a chemistry class. The method we use to display these changes in state versus the conditions is called a phase diagram. Before we start analyzing a phase diagram we must first understand the definitions that are used to talk about actions on the phase diagram. We want to know all the words to describe each change in state. All those words are contained in the picture below.
This picture above suggests that it is possible to go directly from solid to gas state without going through the liquid state. The best example of this situation is dry ice. Click the link to see it.
Examples: Give the name for each specific change in state.
| From liquid to solid | freezing |
| From gas to liquid | condensation |
| From liquid to gas | vaporization |
PRACTICE PROBLEMS: Give the name for each specific change in state.
| From solid to liquid | melting |
| From gas to solid | deposition |
| From solid to gas | sublimation |
Chem – Pressure
What is pressure?
The scientific definition of pressure is force divided by area. Again this definition is not that great for giving you an idea of how to think about pressure. Luckily humans have built in experience with pressure so I will use that as an example. We measure the pain we feel in terms of pressure. The higher the pressure the more painful the lower the pressure the less painful. So if you put your whole hand on a table and push down with the weight of your body it is low to zero pain on your hand (low pressure). However, if you put just the tip of one of your fingers down on the table and push down with the weight of your body then you feel lots of pain in your finger (high pressure). What changed in that situation? You had the same weight or mass pressing down, but the amount of area of your body that was in contact with the table was different. When you had a large amount of area touching the table (whole hand) it was not very painful but when you had a tiny area touching the table (one finger) it was very painful. This is the demonstration of force divided by area.
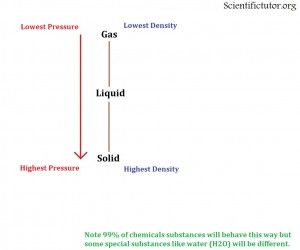
How does the pressure affect the state of a chemical?
To think of pressure in terms of chemistry we want to ask ourselves again what we are doing to the molecules. If we increase the pressure on two molecules (like in a gas state) we are squeezing them together. In other words:
The HIGHER the pressure on a chemical the CLOSER the molecules are pushed together.
The LOWER the pressure on a chemical the FURTHER the molecule are pushed apart.
Therefore, based on our knowledge from the molecules and states of matter section we can suggest how the state of matter of a chemical will be affected by pressure.
If we constantly drive the PRESSURE HIGHER then that creates LESS SPACE between the molecules and they will eventually be in a SOLID STATE.
If we constantly drive the PRESSURE LOWER then that creates MORE SPACE between the molecules and they will eventually be in a GAS STATE.
Below I have created a simple picture to describe this situation.
Chem – Temperature
What is temperature?
A lot of people have trouble with this definition in chemistry classes because they are given a long and complicated definition. I will give the complete definition of temperature first and then attempt to simplify it for you. What I want to point out before hand is that temperature is a measurement. Temperature is measuring the average kinetic energy of the molecules of a substance (solid, liquid, or gas). Usually at this point teachers and books go on to explain more about kinetic energy with a mathematical equation. I am not a fan of that, and you do not need to know that mathematical equation for chemistry. However, if you want that explanation the link is there. To put it simply, kinetic energy has two variables (things) that can effect it. That is the mass of the molecule (particle) or the velocity (speed) of a molecule (particle). For gases, if we assume all of them have about the same mass, we can deduce that the velocity (speed) at which they are traveling is the main contributor to their kinetic energy. Therefore, since temperature measures kinetic energy and kinetic energy is mostly determined by the speed of the molecule, it is not too much of an assumption to say that temperature is mainly a measurement of how fast molecules (particles) are moving.
The HIGHER the temperature the FASTER molecules are moving.
The LOWER the temperature the SLOWER molecules are moving.
How does temperature affect the state of a chemical?
As molecules speed up their vibrations or collisions this creates more space between one molecule and another. This is much like the situation when you have a crowd of people and one person starts violently flailing or moving quickly the crowd around them moves further away because they are pushed further away. As we continue to create more space between molecules they become closer to the gas state. Lets review what we just said.
If we constantly drive the TEMPERATURE HIGHER then that creates MORE SPACE between the molecules and they will eventually be in a GAS STATE.
If we constantly drive the TEMPERATURE LOWER then that creates LESS SPACE between the molecules and they will eventually be in a SOLID STATE.
Below I have created a simple picture to describe this situation.
Chem – The Formation of Different States of Matter
How do different states of matter form? Why do different states of matter form?
VIDEO explanation of the formation of states of matter.
The different states of matter form because of the specific conditions of chemicals in that part of the universe. Where we most commonly interact with matter, our planet Earth, the different states form mainly because of the amount of gravity that the earth generates. This gravity is sorting the chemicals on our planet like the rock, ocean, and air by its density. The more dense a chemical is the closer to the center of our planet it will start to move. It is easy to see this sorting of density by standing at the shore of a lake or beach. This gives you a peak at the layering by density of the Earth. The air is the least dense material so it is on the top layer (above the water). The water is of middle density so it is between the air and the ground (ocean or lake bottom). The rock or sand is the most dense since it is below the water. On the surface of Earth we can make a pretty simple distinction between the different phases and how they correspond to different density sorting by gravity.
Chem – Aqueous
What is the aqueous state of matter?
Simply put aqueous means mixed with water. Some chemicals mix with water and others do not. Miscible means that two chemicals (like water and something else) are able to mix. Immiscible means that two chemicals are not able to mix. The other term for mixing together chemicals is solubility. How soluble two chemicals are when mixed together means how well or how fast do they mix. Two chemicals (like water and something else) that are very soluble will mix together very well and very fast. Two chemicals that are slightly soluble will mix together very poorly and very slowly. You are probably most familiar with the mixing of common table salt ( NaCl ) in water this is one example of an aqueous mixture. That means the salty ocean is an example of an aqueous state. However, gases can also be mixed with water to form an aqueous state. You can see this in a soda like Coke. CO2 gas is mixed with water and when they are in an aqueous state (fizzy) you can observe a taste and texture change. The sodas taste and texture changes when the CO2 is no longer mixed in (flat).
(Pic aqueous state of matter)
What is important about the aqueous state?
Aqueous states are important because they have some special properties. Both the water and the other chemical ( like NaCl ) that are mixed together can affect each other. The pressure characteristics of the water are affected by the salt ions and the state of the salt is changed from a solid form (the salt dissolves). Aqueous states also affect the freezing point and boiling point of the liquid. Lastly, there are limits to how much of the gas or liquid you can dissolve in the liquid and crystals or bubbling start to form when these limits are reached. Most of the above properties are explored in the solutions lesson but we will explore all of these special properties of aqueous solutions by the time chemistry is over.
Chem – Molecules and States of Matter
What are the states of matter?
VIDEO Molecules and States of Matter
The states of matter that we commonly interact with are solid, liquid, and gas. An example of a solid is the wood of a tree. An example of a liquid is the water that you drink. An example of a gas the air that you breath. What are the differences between these states on a small scale (molecular level)? Before we answer that question we need of a good way to think of molecules. The best way to think of a molecule at any state is like a tiny sphere shaped object. We can then picture how two molecules (two spheres) interact in two critical ways. 1) The amount of space between two molecules of the same state and 2) How two molecules of the same state move next to or around each other. First let us talk about the amount of space.
What are the differences between the states of matter?
If you have two molecules of SOLID they are a SHORT distance from one another. If you have two molecules of LIQUID they are a MEDIUM distance from each other. If you have two molecules of GAS they are a LARGE distance from one another. Now lets us talk about how the molecules move next to or around each other. Two solid molecules will only be allowed to vibrate next to each other and bump one another. Solid molecules cannot pass around or between any of the neighboring molecules. Two liquid molecules can vibrate next to each other but they also have the ability to tumble over and past each other. Two gas molecules can vibrate next to each other but they also have the ability to tumble over each other or violently bounce off one another to move away from their neighbor at tremendous speeds. In fact gas molecules are always in motion, shooting away from and knocking into one another similar to soccer players on a field. This description of how molecules move relative to one another is what is called the Kinetic Molecular Theory. Lets break down the word Kinetic Molecular to give ourselves a simple description. Kin or Kinetic means the movement or motion of. Molecular means molecule. So the Kinetic Molecular Theory simply describes the movement or motion of molecules. There is one state of matter that we did not describe here that they do talk a lot about in chemistry. That state is Aqueous represented by ( aq ).
Video demonstration of how solid molecules act
Video demonstration of how liquid molecules act
Video demonstration of how gas molecules act
Chem – LESSON 9: States of Matter
What is the lesson about?
This lesson is about how the properties of the small molecules relate to the properties of the large objects we interact with. We can learn how to manipulate these properties to our advantage in order to produce the exact state of any chemical we desire. At a gym we can go from a steam room to swimming pool to drinking a cup of water with ice in it. All of those were examples of one chemical H2O, but each of them had the H2O in a different state.
Why is it critical to understand?
It is critical to understand how the individual molecules of a larger picture work together in order to give a strong foundation in the concept thinking that is needed for all future lessons. Without this you will not be able to think about why things happen like they do in lessons like chemical equations, gas laws, solutions, and equilibrium. If you miss the fundamental understanding of how matter works and forms then the rest of chemistry will seem like a random string of pointless formulas and concepts that have nothing to do with each other. Create a mental video from this lesson that will carry you through how everything is related to the interactions of the different molecules.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to sections on
New Learning Sections:
—> Molecules and States of Matter
—> Aqueous
—> The Formation of Different States of Matter
—> Temperature
—> Pressure
—> Words Used to Describe Transitions Between States of Matter
—> College: Molar Mass and States of Matter
—> College: Molecular Forces and States of Matter
—> College: Different Types of Solids
Reference Pages:
Worksheets:
Chem – Intermolecular Forces from Lewis Structures
What are intermolecular forces?
Intermolecular forces (or bonds) are the forces that hold together two different molecules. For example, the forces that hold together two H2O molecules to each other. Intermolecular forces are the reason why when water comes out of the end of a faucet or a squirt gun it stays together in a stream and does not fly apart in every direction.
How do you determine the intermolecular forces of a molecule or chemical?
To determine the intermolecular forces of a molecule or chemical, you must first construct the electron dot structure (Lewis structure). Then you determine the polarity of the molecule. Once you have those done determining the types of intermolecular bonds become much more simple. NON-POLAR molecules have only the strongest intermolecular bonds of LONDON DISPERSION. Polar molecules can either have the intermolecular forces of HYDROGEN BONDING or DIPOLE DIPOLE.
What is the difference between Hydrogen Bonding and Dipole Dipole?
The difference between hydrogen bonding and dipole dipole is that hydrogen bonding is a special strong type of dipole dipole. We can tell a HYDROGEN BONDING molecule because it will have within it a bond between H and F or between H and O or between H and N. If it does not have any of those bonds within the molecule, then it will be a Dipole Dipole. I will illustrate this in the table below:
(Intermolecular forces table)
Examples: Name the strongest intermolecular force between the two identical molecules from each question below. VIDEO Determining Intermolecular Forces from Electron Dot Structure Examples 1.
Between SF4 and SF4
Electron dot structure picture with polarity if needed.
ANSWER: London Dispersion
Between H2O and H2O
Electron dot structure picture with polarity if needed.
ANSWER: Hydrogen Bonding
Between PCl3 and PCl3
Electron dot structure picture with polarity if needed.
ANSWER: Dipole Dipole
Between H2CO and H2CO
Electron dot structure picture with polarity if needed.
ANSWER: Dipole Dipole
PRACTICE PROBLEMS: Name the strongest intermolecular force between the two identical molecules from each question below.
Between SH2 and SH2
Electron dot structure picture with polarity if needed.
ANSWER: Dipole Dipole
Between I2 and I2
Electron dot structure picture with polarity if needed.
ANSWER: London Dispersion
Between NH3 and NH3
Electron dot structure picture with polarity if needed.
ANSWER: Hydrogen Bonding
Between HCN and HCN
Electron dot structure picture with polarity if needed.
ANSWER: Dipole Dipole
Between CO2 and CO2
Electron dot structure picture with polarity if needed.
ANSWER: London Dispersion
Chem – Bond Polarity and Electronegativity Part 2
NOT YET COMPLETE
Chem – Bond Polarity and Electronegativity Part 1
What is bond polarity?
Bond polarity is the electronegativity difference between two atoms that are bonded. Because of this electronegativity difference, the electrons get pulled more toward one atom. This means one atom becomes partially positive (the atom losing electrons) and the other atom becomes partially negative (the atom gaining the electrons). In this section you want to be able to recognize which atom has the higher electronegativity in a bond between two atoms using an electronegativity table.
Examples: Use the electronegativity table to determine the polarity of the bond. Which side is partially negative and which is partially positive? VIDEO Bond Polarity Examples 1.
(N bonded to O picture)
Answer: N is more positive O is more negative. Picture of Answer
(F bonded to S picture)
Answer: S is more positive F is more negative. Picture of Answer
(P is bonded to Cl pic)
Answer: P is more positive Cl is more negative. Picture of Answer
PRACTICE PROBLEMS: Use the electronegativity table to determine the polarity of the bond. Which side is partially negative and which is partially positive?
(Br is bonded to As pic)
Answer: As is partially positive Br is partially negative. Picture of Answer
(Si is bonded to O pic)
Answer: Si is partially positive O is partially negative. Picture of Answer
(H is bonded to F pic)
Answer: H is partially positive F is partially negative. Picture of Answer
(P is bonded to Al pic)
Answer: Al is partially positive P is partially negative. Picture of Answer
Chem – College: Hybridization
Page Not Yet Complete
Chem – College: Electron Geometry and Steric Number
What is the difference between electron geometry (electron domains / electron clouds / steric number) and molecular shape (molecular geometry)?
Both are very similar, but the difference is in how we treat that electron’s lone pairs versus the bonds. In molecular shape (molecular geometry) you treat the electron’s lone pairs and the bonds as two different and separate things. However, with electron geometry (steric number) lone pairs and bonds are treated the same. You also treat double and triple bonds as one group instead of 3 just like you did in molecular shape. This means you count up the lone pairs and number of atoms attached to the central atom into one number (the steric number) and from there determine electron geometry. Refer to the electron geometry table below.
Examples: Use the electron geometry table to determine the steric number and electron geometry of each molecule. VIDEO Electron Geometry Examples 1.
(pic SO2)
What is the steric number and electron geometry of SO2?
ANSWER: 3……Trigonal Planar
(pic SH2)
What is the steric number and electron geometry of SH2?
ANSWER: 4……Tetrahedral
(pic BrF3)
What is the steric number and electron geometry of BrF3?
ANSWER: 5……Trigonal Bipyramidal
(pic PH5)
What is the steric number and electron geometry of PH5?
ANSWER: 6……Octahedral
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) and the electron geometry table to determine the steric number and electron geometry.
(pic NH3)
What is the steric number and electron geometry of NH3?
ANSWER: 4……Tetrahedral
(pic NO3-)
What is the steric number and electron geometry of NO3-?
ANSWER: 3……Trigonal Planar
(pic N2)
What is the steric number and electron geometry of N2?
ANSWER: 4……Tetrahedral
(pic H2O)
What is the steric number and electron geometry of H2O?
ANSWER: 4……Tetrahedral
(pic PBr5)
What is the steric number and electron geometry of PBr5?
ANSWER: 6……Octahedral
(pic BrH3)
What is the steric number and electron geometry of BrH3?
ANSWER: 5……Trigonal Bipyramidal
(pic HCN)
What is the steric number and electron geometry of BrH3?
ANSWER: 2……Linear
Chem – College: Molecular Shapes and Bond Angle Continued
What are the more advanced molecular shapes (molecular geometry)?
Before taking on this section, make sure you have gone over the completing the octet college section. Those molecular shapes and bond angle beyond Tetrahedral (4 bonds – or electron clouds) are the more advanced shapes. They include shapes like triginal bipyrimidal and octahedral but only make sense if you have explored the electron dot structures (Lewis structures) beyond the octet of 8. You identify these molecular shapes and bond angles from their electron dot structures by counting both the atoms bonded to the central atom and the lone pair electrons of the central atom. Use the complete molecular shape (molecular geometry) table below to answer the examples and practice problems.
Examples: Use the complete molecular shape (molecular geometry) table along with the electron dot structure (Lewis structure) to determine the molecular shape (molecular geometry). VIDEO Molecular Shape from Lewis Structure Examples 2.
(pic SF6)
Atoms bonded to central atom: 6
Lone pair electrons of central atom: 0 pairs
Bond Angle of SF6 (Answer): 90 degrees
Molecular Shape of SF6 (Answer): OCTEHEDRAL
(pic PH5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 1 pair (2 electrons total)
Bond Angle of PH5 (Answer): 90 degrees
Molecular Shape of PH5 (Answer): SQUARE PYRAMIDAL
(pic AsBr3H2)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 0 pairs
Bond Angle of AsBr3H2 (Answer): 90 and 120 degrees
Molecular Shape of AsCl3H2 (Answer): TRIGONAL BIPYRIMIDAL
(pic BrH3)
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 2 pairs (4 electrons total)
Bond Angle of BrH3 (Answer): Less than 90 degrees
Molecular Shape of BrH3 (Answer): T-SHAPED
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) to determine the molecular shape (molecular geometry). You might need the complete molecular shape (molecular geometry) table.
(pic SeBr6)
Atoms bonded to central atom: 6
Lone pair electrons of central atom: 0 pairs
Bond Angle of SeBr6 (Answer): 90 degrees
Molecular Shape of SeBr6 (Answer): OCTAHEDRAL
(PBr5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 0 pairs
Bond Angle of PBr5 (Answer): 120 and 90 degrees
Molecular Shape of PBr5 (Answer): TRIGONAL BIPYRAMIDAL
(pic KrH2)
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 3 pair
Bond Angle of KrH2 (Answer): 180 degrees
Molecular Shape of KrH2 (Answer): LINEAR
(BrH5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 1 pairs
Bond Angle of BrH5 (Answer): 90 degrees
Molecular Shape of BrH5 (Answer): SQUARE PYRAMIDAL
(KrH31-)
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 3 pair
Bond Angle of KrH31- (Answer): Less than 90 degrees
Molecular Shape KrH31- (Answer): T-SHAPED
(XeF4)
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 2 pairs
Bond Angle of XeF4 (Answer): 90 degrees
Molecular Shape of XeF4 (Answer): SQUARE PLANAR
Chem – Bond Angle
What is the bond angle?
Bond angle is another way to look at the molecular shapes or 3-D structure of a flat 2-D drawn electron dot structure (Lewis structure). It is the angle between any two covalent bonds in an electron dot structure.
How do electron dot structures (Lewis structures) relate to the bond angle?
Bond angle and molecular shape are essentially the same thing, but we are only talking about it from a different perspective. The bond angle is the shortest angle between one bond and another bond. This again is driven by the electrons in the bonds and lone pairs of the central atom repelling each other and trying to maintain the furthest distance apart as possible. The theory of electrons repelling each other to create the shape of the molecule is called the VSEPR (Valence Shell Electron Pair Repulsion) model.
How do you determine the bond angle from the electron dot structure (Lewis structure)?
What you are going to focus on to determine the bond angle of a molecule is the central atom from your electron dot structure. You are going to ask the question, HOW MANY ATOMS ARE ATTACHED TO THE CENTRAL ATOM through bonds AND HOW MANY LONE PAIRS EXIST AROUND ONLY THE CENTRAL ATOM? After that you can refer to the bond angle table below:
Once you add up the number of atoms bonded to the central atom and the number of lone pair electrons around the central atom as two distinct and separate numbers, then you will get your look over at the bond angle column and get your answer.
Examples: Use the electron dot structure (Lewis structure) and the bond angle table to determine the bond angle. VIDEO Bond Angle from Lewis Structure Examples 1.
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 3 pairs (6 electrons total)
Bond Angle of I2 (Answer): 180 degrees
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Bond Angle of SiF4 (Answer): 109.5 degrees
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Bond Angle of H2CO (Answer): 120 degrees
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs (4 electrons total)
Bond Angle of SH2 (Answer): Less than 109.5 degrees
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) and the bond angle table to determine the bond angle.
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Bond Angle of NH4+ (Answer): 109.5 degrees
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Bond Angle of NO31- (Answer): 120 degrees
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 1 pair
Bond Angle of N2 (Answer): 180 degrees
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs
Bond Angle of H2O (Answer): Less than 109.5 degrees
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 1 pair
Bond Angle of PCl3 (Answer): Less than 109.5 degrees
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 0 pairs
Bond Angle of HCN (Answer): 180 degrees
Chem – Molecular Shape (Molecular Geometry)
How do electron dot structures (Lewis structures) relate to the molecular (3 dimensional) shapes?
Many molecules but not all you create by electron dot structures (Lewis structures) exist not a flat two dimensional molecules like you drew them on a piece of paper but occupy 3 dimensions. This is driven by the electrons in the bonds and lone pairs of the central atom repelling each other and trying to maintain the furthest distance apart as possible. The theory of electrons repelling each other to create the shape of the molecule is called the VSEPR (Valence Shell Electron Pair Repulsion) model.
How do you determine the molecular shapes (molecular geometry) from the electron dot structure (Lewis structure)?
What you are going to focus on to determine the molecular shape of a molecule is the central atom from your electron dot structure. You are going to ask the question, HOW MANY ATOMS ARE ATTACHED TO THE CENTRAL ATOM through bonds AND HOW MANY LONE PAIRS EXIST AROUND ONLY THE CENTRAL ATOM? After that you can refer to the molecular shape table below:
Once you add up the number of atoms bonded to the central atom and the number of lone pairs electrons around the central atom as two distinct and separate numbers then you will get you look over at the molecular shape column and get your answer.
Examples: Use the electron dot structure (Lewis structure) and the molecular shape table to determine the molecular shape (molecular geometry). VIDEO Molecular Shape from Lewis Structure Examples 1.
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 3 pairs (6 electrons total)
Molecular Shape of I2 (Answer): LINEAR
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Molecular Shape of SiF4 (Answer): TETRAHEDRAL
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Molecular Shape of H2CO (Answer): TRIGONAL PLANAR
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs (4 electrons total)
Molecular Shape of SH2 (Answer): BENT
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) and the molecular shape table to determine the molecular shape (molecular geometry).
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Molecular Shape of NH4+ (Answer): TETRAHEDRAL
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Molecular Shape of NO3– (Answer): TRIGONAL PLANAR
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 1 pair
Molecular Shape of N2 (Answer): LINEAR
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs
Molecular Shape of H2O (Answer): BENT
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 1 pair
Molecular Shape of PCl3 (Answer): TRIGONAL PYRAMIDAL
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 0 pairs
Molecular Shape of HCN (Answer): LINEAR
NT
Chem – College: Using the Formal Charge
Why is formal charge important?
Formal charge is a way to tell which resonance structures (your different possible electron dot structures) you have crated are best representing what the molecule actually looks like. Their can be multiple possible ways to draw correct electron dot structure (Lewis structures) and formal charge is a quick and easy way to distinguish between them. Are some electron dot structures (Lewis structures) better than others? Yes. Those with the least extreme formal charges or formal charges that are most in line with electronegativity are the best.
Examples: Draw the best electron dot structures (Lewis structures) possible using formal charge to test them. VIDEO Using Formal Charge Examples 1.
| Molecule | Structure |
| N2O | Answer link 1 |
| SO42- | Answer link 2 |
| NO32– | Answer link 3 |
PRACTICE PROBLEMS: Draw the best electron dot structures (Lewis structures) possible using formal charge to test them.
CANNOT THINK OF ANY GOOD PRACTIC PROBLEMS RIGHT NOW
Chem – College: Finding the Formal Charge
What is formal charge?
Formal charge is a technique to compare the original valence electrons of an atom to the number of valence electrons that remain close to each individual atom at the end of forming the electron dot structure (Lewis structure).
How do you determine the formal charge?
To determine the formal charge take the original number of valence electrons and subtract the number of electrons closest to the atom after the electron dot structure (Lewis structure) is complete. To achieve this draw a box around each atom and the electron lone pairs. This box should also cut the bonds to other atoms in half (essentially returning half the electrons in one bond to one atom and half to the other). Do this for each atom in the electron dot structure (Lewis structure).
For each atom on the Lewis Structure:
Original valence electrons – Remaining electrons = Formal Charge
Examples: Give the formal charge of each atom in the Lewis Structure. VIDEO Formal Charge Examples 1.
| Molecule | Structure |
| H2CO | Answer link 1 |
| NH4+ | Answer link 2 |
| NO3– | Answer link 3 |
PRACTICE PROBLEMS: Give the formal charge of each atom in the electron dot structure (Lewis structure).
| Molecule | Structure |
| SiBr4 | Answer link 1 |
| H2O | Answer link 2 |
| CN– | Answer link 3 |
| CO32- | Answer link 4 |
Resonance Structures
VIDEO explanation of resonance structures.
What are resonance structures?
Resonance structures are possible alternatives ways to present or draw electron dot structures (Lewis structures) that have double or triple bonds. Not all electron dot structures (lewis structures) that have double or triple bonds will have resonance structures but many of the ones you come across will. Examples below.
(NO3- pictures)
In the pictures above you can notice that the double bond could form between any of the oxygens and the nitrogen. This means that the structures shown are resonance structures of each other. The double bond can resonate (rotate) to produce any of the possible structures.
Examples: Draw the electron structure and then find all possible resonance structures for the molecule or ion. VIDEO Resonance Structures Examples 1.
| Molecule | Structure |
| NO2– | Answer link 1 |
| CNI | Answer link 2 |
| SiO32– | Answer link 3 |
PRACTICE PROBLEMS: Draw the electron structure and then find all possible resonance structures for the molecule or ion.
| Molecule | Structure |
| P2S | Answer link 1 |
| N3O3- | Answer link 2 |
I am very limited in the practice problems I can present unless I know your class has taught the more complicated college sections on electron dot structures (Lewis structures).
Chem – College: Completing the Octet
What is the expanded octet?
All rules are same in this section for completing the octet but the octets of some elements are more complicated. If you get into the more advanced octets then elements like Boron and Aluminum will form octets of 6 and elements like Phosphorus and Sulfur can form octets between 8 and 12. Below I will lay out the elements according to their octet.
At a college level these are the octets that you have to know:
8: C, Si, N, O, F
2: H
6: B, Al
*8-12: S, P, Cl, Br, I, As, Se
* If you are confused on choosing an octet when they are between 8-12 it is usually better to go for a higher octet. Just practice and do the best you can in this section. However, we will address concerns about how to choose your octet when you have options like 8-12 as we get into other sections of this lesson like finding the formal charge and using the formal charge.
The cross configuration of outer atoms that I have been teaching you so far in this lesson needs to be expanded in this section. Since we can go beyond an octet of 8 then that means we can have more than 4 outer atoms. So try to put the outer atoms as evenly spaced from each other and the central atom as you can. As you do more problems you will get better at it.
Examples: Use the picture in the STARTING PICTURE COLUMN and complete the octet of each atom by drawing the appropriate amount of bonds. VIDEO Completing the Octet Examples 1.
| Molecule | Starting Picture | Structure |
| AlBr3 | AlBr3 Valence Pic | Answer link 1 |
| PH5 | PH5 Valence Pic | Answer link 2 |
| IO31- | IO31- Valence Pic | Answer link 3 |
| SO42- | SO42- Valence Pic | Answer link 4 |
PRACTICE PROBLEMS: Use the picture in the STARTING PICTURE COLUMN and complete the octet of each atom by drawing the appropriate amount of bonds. Remember if you get stuck, start over from the beginning and try again.
MATERIAL NOT YET FINISHED
Chem – Completing the Octet
VIDEO explanation of the Octet and How to Complete it.
WARNING: Many teachers choose to oversimplify the completion of the octet step by instead phrasing it in terms of the number of bonds. They do this because completing the octet is the most difficult step. However, if you oversimplify this step then you will carry with you a handicap that will plague you for the rest of your college based chemistry classes. I am a fan of learning it correct the first time.
What is the octet?
The octet is a concept that all the valence electrons that stay connected to an individual atom will form into some kind of regular predictable pattern. The most common number of predictable valence electrons is 8 which is where the word OCTet comes from (like an OCTopus has 8 arms). Many atoms of elements try to form their valence electrons into groups of 8 by combining (sharing) valence electrons with other atoms. We call this sharing of electrons the creation of bonds.
What are the octets for each element?
Although 8 is the most common octet it is not the only possible octet. The special element Hydrogen also predictably forms an octet of 2. Below I will lay out the elements according to their octet.
At a high school level these are the octets that you have to know:
8: C, Si, N, O, F, Cl, Br, I, S, P
2: H
How do you complete the octet in an Electron Dot Structure (Lewis structure)?
Regardless if atoms are searching for an octet of 8 or 2, the way they do this is by creating bonds. Each bond made is constructed with 2 electrons locking themselves in a particular arrangement to pull the two atoms they are near together. In an actual chemical, the bonds form to trick each atom in the bond partner into thinking they have more electrons in their octet (valence shell) than they actually do. So if carbon and hydrogen form one bond between themselves, then the two electrons contained in that one bond count for both the octets of carbon and the octet of hydrogen. However, lone electrons (non-bonded electrons) do not count for the octet of two atoms. Lone electrons only count for the octet of the atom they are closest to.
To count the octet of each atom you count all lone electrons near that atom plus all the electrons contained in the bonds that are connected to that atom. Each atom must have a complete octet to form an overall correct electron dot structure (Lewis structure). In addition to connecting atoms with bonds to form octets, in some extreme cases you may have to move electrons from one atom to another in order to complete the octet. There is no restriction on when or how many electrons you can move from one atom to another. As long as the octets are correct it should work. The only rule on what not to do in an electron dot structure (Lewis structure) is to not connect the outer atoms to other outer atoms. This will only happen in advanced college organic chemistry classes for reasons that you do not need to know. Visit the organic chemistry area of the website if you are curious but make sure you understand this entire Molecular Structure lesson first.
How do I become successful with the octet?
The technique of applying the octet takes some getting use to so I have set up some rules or guidelines that I have seen be successful over the years I have taught.
1) Represent bonds as lines. Many teachers say to use dots instead of lines for bonds. However, this confuses students when trying to count the octet.
2) When you draw a bond (with a line) immediately delete or draw over the 2 electrons that the bond is representing. The most common mistake I see is for people to lose track of how many valence electrons they start with but drawing bonds and then forgetting to erase the electrons that made them or erasing electrons to move them and then never drawing them back in.
3) Complete the octet of the outer atoms first. Most people try and solve the octet of the central atom first. It is not wrong I have just found that it is harder for people to think in that direction and creates less success for them.
4) Only form one bond at a time and then count the octet. The faster people try to form the octet the more likely they are to make a mistake. People try and form multiple bonds and then end up with an octet that is too high and become paralyzed with confusion.
5) If you become confused and don’t think you can continue to move forward to complete the octet of any atom then stop and start over from the beginning again. The second most common reason while people cannot finish an electron dot structure (Lewis structure) is they think there is only one way to do it. This causes them to repeat the same steps over and over again while trying the same electron dot structure (Lewis structure) multiple times and never getting an answer that satisfies them.
6) If one set of steps did not work try something completely different next time. Changing which order you complete the octet in or which direction you draw the electron dot structure (Lewis structure) in can change your ability to solve for the correct structure. People get stuck thinking one way and it can cause them to miss how to make things easier or better for themselves. Shake things up. The worst that can happen is you do no better than you did last time.
7) Have the rules for the octets of each element next to you. This is to prevent possible frustration because you thought an elements octet was different than it actually was.
8) Be patient and keep trying. The more you practice and the more you attempt to solve problems the more you will learn and the better you will become at electron dot structures (Lewis structures). Just because you didn’t finish doesn’t mean you didn’t learn anything.
Examples: Use the picture in the STARTING PICTURE COLUMN and complete the octet of each atom by drawing the appropriate amount of bonds. VIDEO Completing the Octet Examples 1.
| Molecule | Starting Picture | Structure |
| SiF4 | SiF4 Valence Pic | Answer link 1 |
| H2CO | H2CO Valence Pic | Answer link 2 |
| SH2 | SH2 Valence Pic | Answer link 3 |
| I2 | I2 Valence Pic | Answer link 4 |
| NH41+ | NH41+ Valence Pic | Answer link 5 |
| NO31- | NO31- Valence Pic | Answer link 6 |
PRACTICE PROBLEMS: Use the picture in the STARTING PICTURE COLUMN and complete the octet of each atom by drawing the appropriate amount of bonds. Remember if you get stuck, start over from the beginning and try again.
| Molecule | Starting Picture | Structure |
| SiBr4 | SiBr4 Valence Pic | Answer link 1 |
| HCN | HCN Valence Pic | Answer link 2 |
| PCl3 | PCl3 Valence Pic | Answer link 3 |
| N2 | N2 Valence Pic | Answer link 4 |
| H2O | H2O Valence Pic | Answer link 5 |
| CN1- | CN1- Valence Pic | Answer link 6 |
| CO32- | CO32- Valence Pic | Answer link 7 |
Covalent Bonds in Electron Dot Structures (Lewis Structures)
VIDEO explanation of the Covalent Bonds in an Electron Dot Structure (Lewis structure).
What is a covalent bond?
All following steps in creating and thinking about electron dot structures (Lewis structures) require that we first understand what a covalent bond is. When valence electrons from different atoms come near each other they can form covalent bonds by sharing their electrons in an orbital between them. That means the electrons travel back and fourth between the two atoms. The picture below represents two atoms with their valence electrons just before they are about to join in a covalent bond.
How do we represent covalent bonds in Electron Dot Structures (Lewis structures)?
Each single covalent bond is made up of 2 electrons.
We can represent a covalent bond in an electron dot structure (Lewis structure) by a blue line that connects the 2 valence electron red dots in our pictures. An example is below.
You can also create electron dot structures (Lewis structures) with more than one bond. This is known as creating double bonds or triple bonds. A double bond is just two bonds created between the same two atoms in an electron dot structure (Lewis structure). Example below.
A triple bond is just three bonds created between the same two atoms in an electron dot structure (Lewis structure). Example below.
PRACTICE PROBLEMS: Try drawing for yourself single, double, and triple bonds in structures. Start with the picture I have provided and then complete it.
Cl2 has a single bond between the two atoms.
Se2 has a double bond between the two atoms.
As2 has a triple bond between the two atoms.
Chem – Filling in the Valence Electrons of an Electron Dot Structure (Lewis Structure) that is an Ion
How to do fill in valence electron dots for an Electron Dot Structure (Lewis structure) that is an ion?
Filling in valence electrons also for electron dot structures (Lewis structures) also requires us to take a look at special cases where how many valence electrons are in a covalent molecule might change. Those special cases are called ions. More specifically we will be working with polyatomic ions. These polyatomic ions will form an electron dot structure (Lewis Structure) but will have some valence electrons added to them or some taken away. This addition or subtraction of valence electrons is indicated by their charge. Where you add or subtract valence electrons while drawing an electron dot structure (Lewis Structure) in theory does not matter. However in practice, I have found that it is easier for people to understand if they subtract electrons from the central atoms or add electrons to the outer atoms.
Examples: Use the picture in the STARTING PICTURE COLUMN and then fill in the valence electrons as dots around the atoms they come from. If needed you can look up how many valence electrons each element has with this valence electron periodic table. VIDEO Filling in Valence Electrons of an Ion Examples 1.
| Molecule | Starting Picture | Structure |
| NH4+ | NH4+ Cross Pic | Answer link 1 |
| NO3– | NO3– Cross Pic | Answer link 2 |
| CO22- | CO22- Cross Pic | Answer link 3 |
PRACTICE PROBLEMS: Use the picture in the STARTING PICTURE COLUMN and then fill in the valence electrons as dots around the atoms they come from. Try to remember how many valence electrons each element has from a regular periodic table. If needed you can look up how many valence electrons each element has use this valence electron periodic table.
| Molecule | Starting Picture | Structure |
| OH– | OH– Cross Pic | Answer link 1 |
| CN– | CN– Cross Pic | Answer link 2 |
| H3O+ | H3O+ Cross Pic | Answer link 3 |
| CO32- | CO32- Cross Pic | Answer link 4 |
| SO42- | SO42- Cross Pic | Answer link 5 |
Chem – Filling in the Valence Electrons of an Electron Dot Structure (Lewis Structure)
How to do fill in valence electron dots for an Electron Dot Structure (Lewis structure)?
To fill in the valence electrons for electron dot structures (Lewis structures) make sure you remember the section on how to count valence electrons on the periodic table. Once you know that then you can move on with this section. In electron dot structures (Lewis structures), valence electrons are represented by the dot mark of a pen much like a period at the end of a sentence. Each one dot represents one valence electron. Once you have your cross configuration drawn for your electron dot structure (Lewis structure) you should go to each atom of each element and draw in the valence electrons dots of each atom. You want to place the dots around each atom either above or below or to the right or left of the letter symbol for the atom. Try to distribute the dots as evenly as possible around the letter representing the atom. Usually you will see teachers start to group the valence electron dots in pairs if they have more than 4. Do this for every atom in the Lewis structure you are working on at the time.
Examples: Use the picture in the STARTING PICTURE COLUMN and then fill in the valence electrons as dots around the atoms they come from. If needed you can look up how many valence electrons each element has with this valence electron periodic table. VIDEO Filling in Valence Electrons Examples 1.
| Molecule | Starting Picture | Answers |
| SiF4 | SiF4 Cross Pic | Answer link 1 |
| H2CO | H2CO Cross Pic | Answer link 2 |
| SH2 | SH2 Cross Pic | Answer link 3 |
| I2 | I2 Cross Pic | Answer link 4 |
PRACTICE PROBLEMS: Use the picture in the STARTING PICTURE COLUMN and then fill in the valence electrons as dots around the atoms they come from. Try to remember how many valence electrons each element has from a regular periodic table. If needed you can look up how many valence electrons each element has use this valence electron periodic table.
| Molecule | Starting Picture | Structure |
| SiBr4 | SiBr4 Cross Pic | Answer link 1 |
| HCN | HCN Cross Pic | Answer link 2 |
| PCl3 | PCl3 Cross Pic | Answer link 3 |
| N2 | N2 Cross Pic | Answer link 4 |
| H2O | H2O Cross Pic | Answer link 5 |
Chem – Drawing Atoms in a Cross Shape
How is it best to arrange the atoms on a page in order to draw an Electron Dot Structure (Lewis structure)?
VIDEO explanation of Drawn Out Atoms of an Electron Dot Structure in a Cross Shape.
When I talk about a cross shape I think about a four way street sign. The central atom will be at the point where all the lines meet. The rest of the atoms (the non-central atoms) will be at the ends of each point on the cross. So if we draw SiF4 for example, Si is the central atom. I have demonstrated it in the picture below.
Sometimes we do not have enough atoms in the electron dot structure (Lewis structure) to go all the way around the cross and put atoms at every point. That is the case for electron dot structures (Lewis structures) like H2CO and SH2 and I2 . If this happens just try to fill in as many points (ends) of the cross that you can and forget about the rest. It does not matter which order you fill them in. The pictures of H2CO and SH2 and I2 are demonstrated in below.
PRACTICE PROBLEMS: Draw the cross configuration for the following electron dot structures (Lewis Structures). Be aware that I only show one answer of how to draw them. There can be many different ways to write the answers.
| Molecule | Structure |
| SiBr4 | Answer link 1 |
| HCN | Answer link 2 |
| PCl3 | Answer link 3 |
| N2 | Answer link 4 |
| H2O | Answer link 5 |
Chem – Finding the Central Atom
How do I find the central atom of an Electron Dot Structure (Lewis Structure)?
Many different teachers have many different styles for teaching this first step. I will show you the three I like most and then I will let you choose. Method 1: The easiest to teach and understand is have you memorize a list of elements. That list is; C, Si, N, P, S, O. These elements are also in priority order. So if you have covalent compound with C and P then C is the central atom because it comes first on the list. This list will cover your needs on about 95% of questions you encounter for electron dot structures (Lewis Structures). I usually prefer to teach my students this way at first to just get them rolling in the right direction. Later we can discuss the exceptions or elements that fall outside of the list. Method 2: the element that is the least numerous in the compound. That means if you have something like BF3, then the B is the central atom. I understand why people use this method, it is so easy to think about, but the problem becomes that many electron dot structures (Lewis structures) will have only one of several different elements. For Example, HCN. Which one is the central? With method 2 you cannot tell. Method 2 will cover your needs about 80% of the time. Method 3: the element with the lowest electronegativity will be the central atom (The exception is H. Hydrogen will never be the central atom except in the molecule H2.). I love this method, but it requires that the student knows and understands electronegativity. So if the student has forgotten about electronegativity or has trouble remembering the electronegativity trend then it will be a difficult method for them to use. This method will cover your needs about 99% of the time.
Examples: Find the central atom in the electron dot structure (Lewis Structure) using the method you find best. Here is an Electronegativity Table. VIDEO Finding the Central Atom of an Electron Dot Structure (Lewis Structure) Examples.
| Lewis Structure | Central Atom |
| SiF4 | Si |
| H2CO | C |
| SH2 | S |
| I2 | I |
PRACTICE PROBLEMS: Find the central atom in the electron dot structure (Lewis Structure) using the method you find best. Here is a regular periodic table but you can use an Electronegativity Table if you must.
| Lewis Structure | Central Atom |
| SiBr4 | Si |
| HCN | C |
| PCl3 | P |
| N2 | N |
| H2O | O |
Chem – Putting Together an Electron Dot Structure (Lewis Structure)
What are Electron Dot Structures (Lewis Structures)?
The electron dot structures (Lewis structure) is a way to represent a 3 dimensional covalent chemical on a 2 dimensional piece of paper so that we can better or more easily understand what the properties of that molecule are. It is just like an engineer or architect making a sketch on paper of their eventual 3D building. This section will focus on how we put them together by drawing them on a piece of paper for the purposes of solving chemistry problems. BEFORE YOU START the rest of the sections in this lesson, make sure you understand what valence electrons are. You can review valence electrons from an earlier lesson with these two sections: valence electrons and drawing valence electrons around an atom.
Since valence electron dot structures (Lewis Structures) can sometimes be difficult, I have had to search for a way to be able to teach them simply yet correctly. The rules that I have come up with are listed below. They are listed in the order in which they should be performed. In other words, use rule number 1 first, then 2, then 3, then 4.
What does all this mean? I will go over how to apply each rule in the sections to follow. For some students these rules can seem frustrating at first and to tell you the truth they are. I have seen a lot of teachers and students alike try to take shortcuts with the rules. It always ends up blowing up in their face because it leads them in the wrong direction in the future. If there were a better and faster way I would show you so just keep practicing.
Chem – Lesson 8: Valence Electron Dot Structures (Lewis Structures)
What is the lesson about?
We already learned how ionic compounds form in a previous lesson. The Electron Dot Structures (Lewis Structures) Lesson is about how covalent molecules (non-metal compounds) form. A person named Lewis is the one that described this and that is why it has the name Lewis structures.
Why is it critical to understand?
With a complete education about electron dot structures (Lewis structures), you become very powerful with chemistry. That is to say you can predict how covalent compounds will chemically act just by looking at how they are put together. Valence electron dot structures (Lewis structures) explains things like why water and oil will not mix or why water will form small beads on some surfaces but not others or why oxygen can so easily move from your lungs to your blood stream. In AP and college courses, Electron Dot Structures (Lewis structures) are extremely important to know. This lesson is also the foundation into what is know as organic chemistry. If you do not do well in the electron dot structures (Lewis structures) lesson then it is not possible to pass any lesson or class in organic chemistry.
What you should know before attempting this lesson?
If you have trouble in this lesson go back to the sections Valence Electrons and Drawing Valence Electrons Around an Atom.
New Learning Sections:
—> Putting Together an Electron Dot Structure (Lewis Structure) Part 1
—> Finding the Central Atom Part 2
—> Drawing Atoms in a Cross Shape Part 3
—> Filling in the Valence Electrons of an Electron Dot Structure (Lewis Structure) Part 4
—> Covalent Bonds in Electron Dot Structures (Lewis Structures) Part 6
—> Completing the Octet Part 7
—> College: Completing the Octet Continued
—> College: Finding the Formal Charge
—> College: Using the Formal Charge
—> Molecular Shapes (Molecular Geometry)
—> Bond Angle
—> College: Molecular Shapes and Bond Angle Continued
—> College: Electron Geometry and Steric Number
—> College: Hybridization (sp, sp2, sp3)
—> Bond Polarity and Electronegativity Part 1
—> Bond Polarity and Electronegativity Part 2
—> Molecular Polarity (Polar or Non-Polar Molecules)
—> Intermolecular Forces from Electron Dot Structures (Lewis Structures)
Reference Pages:
—> Valence Electron Dot Structure (Lewis Structure) Rules
—> College: Complete Octet Rules
—> Simple Molecular Shapes (Molecular Geometry) and Bond Angle
—> College: Complete Molecular Shapes (Molecular Geometry) and Bond Angle
—> College: Electron Geometry and Steric Number
—> Electronegativity Difference and Types of Bonds Rules
—> Intermolecular Forces Rules
Worksheets:
Chem – Naming Ionic Compounds with Polyatomic Ions Part 2
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
—> Introduction to Polyatomic Ions Part 1
How do you use an ionic name to create the ionic compound with transition metals?
Now let’s try to reverse the question of the problems. Given the chemical formula come up with the name of the ionic compound with polyatomic ions.
Examples: Given the chemical compound, create the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it. Ionic Naming With a Polyatomic Ions Examples Video 2.
| CsNO3 | Caesium Nitrate |
| Ca(OH)2 | Calcium Hydroxide |
| VCO3 | Vanadium (II) Carbonate |
VIDEO Ionic Naming Demonstrated Example 7: Given the chemical compound, create the name using a regular periodic table. Don’t forget to use the ion periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it.
Fe2(CO2)3
Step 1:
What is the polyatomic ion?
Answer: CO2
| CO2 | ||
| 1 | ||
| 1 | ||
| Total = |
Step 2:
What is the name of the polyatomic ion?
Answer: Carbonite
Step 3:
What is the individual charge of the polyatomic ion?
Answer: -2
| CO22- | ||
| 1 | ||
| 1 | ||
| Total = |
Step 4:
How many of the polyatomic ions are there?
Answer: 3
| CO22- | ||
| CO22- | ||
| CO22- | ||
| Total = |
Step 5:
What is the total charge of the polyatomic ions?
Answer: -6
| CO22- | ||
| CO22- | ||
| CO22- | ||
| Total = | -6 |
Step 6:
What is the name for the symbol Fe?
Answer: Iron
| Fe | CO22- | |
| CO22- | ||
| CO22- | ||
| Total = | -6 |
Step 7:
What is the total charge of the compound?
Answer: +6 -6 = zero
| Fe | CO22- | |
| CO22-– | ||
| CO22- | ||
| Total = | +6 | -6 |
Step 8:
How many Fe atoms?
Answer: 2
| Fe | CO22- | |
| Fe | CO22- | |
| CO22- | ||
| Total = | +6 | -6 |
Step 9:
What is the charge of an individual Fe ion?
Answer: +6 / 2 = +3
| Fe3+ | CO22- | |
| Fe3+ | CO22-– | |
| CO22- | ||
| Total = | +6 | -6 |
Step 10:
What is the Roman numeral that goes with the iron ion?
Answer: III (because it is +3)
Step 11:
What is the complete name of the compound?
COMPLETE ANSWER: Iron (III) Carbonite
PRACTICE PROBLEMS: Given the chemical compound, create the name using a regular periodic table. Don’t forget to use the ionic periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it.
| LiC2H3O2 | Lithium Acetate |
| BaSO4 | Barium Sulfate |
| Rb2SO3 | Rubidium Sulfite |
| WPO4 | Tungsten (III) Phosphate |
| CoCO3 | Cobalt (II) Carbonate |
| NH4Cl | Ammonium Chloride |
Chem – Naming Ionic Compounds with Polyatomic Ions Part 1
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
—> Introduction to Polyatomic Ions Part 1
How do you name ionic compounds with polyatomic ions?
The last hurdle to tackle in ionic compounds has to do with polyatomic ions. These examples and practice problems work in the same ways as ionic compounds in the sections just before this one. All the same rules apply.
Examples: Given the name create the chemical compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it. VIDEO Ionic Naming With a Polyatomic Ions Examples 1.
| Sodium Nitrate | NaNO3 |
| Potassium Sulfite | K2SO3 |
| Iron (III) Carbonate | Fe2(CO3)3 |
VIDEO Ionic Naming Demonstrated Example 6: Given the name create the chemical compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it.
Beryllium Sulfite
Step 1:
What is the polyatomic ion?
Answer: Sulfite
Step 2:
What are the symbols for the polyatomic ion?
Answer: SO3
| SO3 | ||
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the charge of the polyatomic ion?
Answer: -2
| SO32- | ||
| 1 | ||
| 1 | ||
| Total = |
Step 4:
What is the symbol for beryllium?
Answer: Be
| Be | SO32- | |
| 1 | ||
| 1 | ||
| Total = |
Step 5:
What is the charge for beryllium?
Answer: +2
| Be2+ | SO32- | |
| 1 | ||
| 1 | ||
| Total = |
Step 6:
What is the common denominator of 2 and 2?
Answer: 2
| Be2+ | SO32- | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 7:
How much sulfite do you need to add up to -2?
Answer: 1
Step 8:
How much beryllium do you need to add up to +2?
Answer: 1
| Be2+ | SO32- | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 9:
What is the chemical formula for Beryllium Sulfite?
COMPLETE ANSWER: BeSO3
PRACTICE PROBLEMS: Given the name of the chemical compound create the chemical formula using a regular periodic table. Don’t forget to use the ion periodic table if you need it. You can also refer to the poly atomic ion sheet if you need it.
| Copper (I) Hydroxide | CuOH |
| Magnesium Phosphate | Mg3(PO4)2 |
| Zinc (II) Nitrite | Zn(NO2)2 |
| Ammonium Oxide | (NH4)2O |
| Sodium Carbonite | Na2CO2 |
| Iron (III) Cyanide | Fe(CN)3 |
When you finish the lesson, try out the worksheet on nomenclature.
Chem – Naming Ionic Compounds with Transition Metals Part 2
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
How do you use an ionic name to create the ionic compound with transition metals?
Now let’s try answering the problems in the other direction (having the ionic name and creating the compound). Remember to count up all the charges and total them to zero.
Examples: Give the chemical formula of each compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it. VIDEO Ionic Naming With a Transition Metal Examples Video 2.
| Nickle (I) Fluoride | NiF |
| Iron (III) Phosphide | FeP |
| Silver (II) Bromide | AgBr2 |
VIDEO Ionic Naming Demonstrated Example 5: Give the chemical formula of each compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
Gold (II) Nitride
Step 1:
Which element is nitride?
Answer: Nitrogen
Step 2:
What is the symbol for nitrogen?
Answer: N
| N | ||
| 1 | ||
| 1 | ||
| Total = |
Step 3:
What is the charge for nitrogen?
Answer: -3
| N3- | ||
| 1 | ||
| 1 | ||
| Total = |
Step 4:
What is the symbol for gold?
Answer: Au
| Au | N3- | |
| 1 | ||
| 1 | ||
| Total = |
Step 5:
What is the charge on gold?
Answer: +2
| Au2+ | N3- | |
| 1 | ||
| 1 | ||
| Total = |
Step 6:
What is the common denominator of 3 and 2?
Answer: 6
| Au2+ | N3- | |
| 1 | ||
| 1 | ||
| Total = | +6 | -6 |
Step 7:
How much nitrogen do you need to add up to -6?
Answer: 2
| Au2+ | N3- | |
| 1 | N3- | |
| 1 | ||
| Total = | +6 | -6 |
Step 8:
How much gold does it take to add up to +6?
Answer: 3
| Au2+ | N3- | |
| Au2+ | N3- | |
| Au2+ | ||
| Total = | +6 | -6 |
Step 9:
What is the complete chemical compound?
COMPLETE ANSWER: Au3N2
PRACTICE PROBLEMS: Give the chemical formula of each compound using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
| Titanium (I) Chloride | TiCl |
| Cobalt (II) Sulfide | CoS |
| Tin (IV) Oxide | SnO2 |
| Copper (I) Selenide | Cu2Se |
| Iron (II) Phosphide | Fe3P2 |
| Lead (IV) Nitride | Pb3N4 |
Chem – Naming Ionic Compounds with Transition Metals Part 1
What sections should I know before attempting to learn this section?
—> Covalent, Ionic, and Metallic Bonds
—> Breaking Apart Ionic Compounds
How do you name ionic compounds with transition metals?
From this point, we also have to tackle ionic compounds that are made up of what are called transition metals. These metals are roughly in the center of the periodic table and do not always have the same charge. First, we will work with problems where you are given the compound, and you have to answer what the name is.
When naming a compound that contains transition metals, a roman numeral is added in parenthesis to indicate what the charge of the transition metal is. Since metals always have a positive charge, you can always assume that the Roman numeral means a positive number for the charge.
Examples: Create the name from the chemical formula using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
VIDEO Ionic Naming With a Transition Metal Examples 1.
| CuCl | Copper (I) Chloride |
| FeO | Iron (II) Oxide |
| Cr2S3 | Chromium (III) Sulfide |
VIDEO Ionic Naming Demonstrated Example 4: Create the name from the chemical formula using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
TiS
Step 1:
What is the name for symbol S?
Answer: Sulfur
Step 2:
How do you change Sulfur it into the two element naming system?
Answer: Sulfide
Step 3:
What is the charge on sulfur?
Answer: -2
| S2- | ||
| 1 | ||
| 1 | ||
| Total = |
Step 4:
What is the name for the symbol Ti?
Answer: Titanium
| Ti | S2- | |
| 1 | ||
| 1 | ||
| Total = |
Step 5:
What does the entire compound add up to?
Answer: +2 -2 = Zero
| Ti | S2- | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 6:
What is the charge on the titanium?
Answer: Titanium = +2
| Ti2+ | S2- | |
| 1 | ||
| 1 | ||
| Total = | +2 | -2 |
Step 7:
What is the Roman numeral for titanium?
Answer: II (because it is +2)
Step 8:
What is the entire name of the compound?
COMPLETE ANSWER: Titanium (II) Sulfide
PRACTICE PROBLEMS: Give the name of the compound from the chemical formula using a regular periodic table. Don’t forget to use the ion periodic table if you need it.
| NiO | Nickle (II) Oxide |
| MnF2 | Manganese (II) Fluoride |
| VS2 | Vanadium (IV) Sulfide |
| FeN | Iron (III) Nitride |
| Zn2Se | Zinc (I) Selenide |
| Cu3P | Copper (I) Phosphide |