Scientific Tutor
Chem – Drawing Atoms in a Cross Shape
How is it best to arrange the atoms on a page in order to draw an Electron Dot Structure (Lewis structure)?
VIDEO explanation of Drawn Out Atoms of an Electron Dot Structure in a Cross Shape.
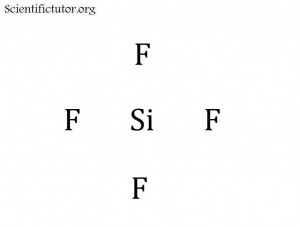
When I talk about a cross shape I think about a four way street sign. The central atom will be at the point where all the lines meet. The rest of the atoms (the non-central atoms) will be at the ends of each point on the cross. So if we draw SiF4 for example, Si is the central atom. I have demonstrated it in the picture below.
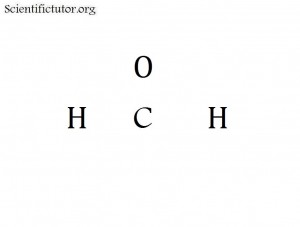
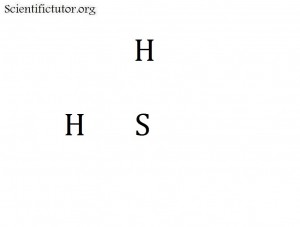
Sometimes we do not have enough atoms in the electron dot structure (Lewis structure) to go all the way around the cross and put atoms at every point. That is the case for electron dot structures (Lewis structures) like H2CO and SH2 and I2 . If this happens just try to fill in as many points (ends) of the cross that you can and forget about the rest. It does not matter which order you fill them in. The pictures of H2CO and SH2 and I2 are demonstrated in below.
PRACTICE PROBLEMS: Draw the cross configuration for the following electron dot structures (Lewis Structures). Be aware that I only show one answer of how to draw them. There can be many different ways to write the answers.
| Molecule | Structure |
| SiBr4 | Answer link 1 |
| HCN | Answer link 2 |
| PCl3 | Answer link 3 |
| N2 | Answer link 4 |
| H2O | Answer link 5 |