Scientific Tutor
Chem – Ionization Energy
What is ionization energy?
For this section, you can open up your ionization energy table with this link. Ionization energy is another of the periodic trends. Ionization energy is the amount of energy it takes to remove an electron from the atom of an element. When they mention this, they are usually talking about the outermost electron or they will call it the first ionization energy. Although that is a decent definition, I prefer to think of ionization energy as how difficult it is to remove or how hard do you need to pull in order to remove an electron from the atom of an element. If you imagine that you were able to physically grab just one electron of an element, would you need to pull it really hard or could you just pop it off with a single finger? If you need to pull the electron really hard to remove it from the element that means it has a high ionization energy. Conversely, if the electron is easy to pull off that means it has a low ionization energy.
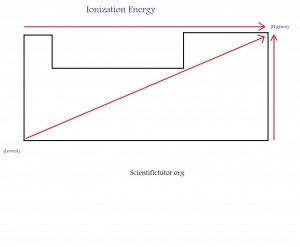
The value (number) for the ionization energy of each element is not really important. You will rarely be asked anything about the values of ionization energy. Instead, most questions will be focused on what is the overall, or general trend, of ionization energy. One trend is ionization energy generally increases as we go from the bottom to the top of the periodic table. It is low at the bottom and high on top. The other trend is ionization energy generally increases as we go from left to right. It is very low on the left side of the periodic table and very high on the right side. Overall, ionization energy is low in the bottom left and high on the top right of the periodic table. If we compare this to electronegativity, we see that the trend is basically the same. The only difference is ionization energy includes the noble gases (group 18) elements whereas, electronegativity does not.Like electronegativity, ionization energy questions are usually asked where they give you about 4 elements and they tell you to pick out the element with the lowest or highest ionization energy. Below is a demonstration in picture form of what I have been describing. The picture is how I like to memorize this trend.
Try a quick sketch of the periodic table with the ionization energy trend at least 3 times to help memorization before you go on to the example and practice problems.
Examples: Without looking at an ionization energy trend (but you can use a regular periodic table), pick out the element with the LOWEST ionization energy. If necessary you can refer to the ionization energy table with this link. VIDEO Comparing Ionization Energy Between Elements Examples 1.
| Ba, Au, Pb, At | Ba |
| N, Sb, As, P | Sb |
| Ag, Si, F, Ca | Ca |
PRACTICE PROBLEMS: Without looking at an ionization energy trend (but you can use a regular periodic table), pick out the element with the HIGHEST ionization energy. If necessary you can refer to the ionization energy table with this link.
| Mg, Cl, Ar, Al | Ar |
| Cr, Se, Ca, Ge | Se |
| Be, Sr, Ba, Mg | Be |
| Br, I, At, F | F |
| Fe, Cs, S, Ni | S |
| Ag, O, Si, Na | O |