Scientific Tutor
Chem – Precipitation Definition and Chemical Equations
What is Precipitation?
It is the act of one substance changing state and therefore removing itself from the solution / mixture.
The change of state will happen when you get a change of conditions (either a change of temperature, pressure, or having chemicals added to it). The most common way you see precipitation is when water molecules in the gas state collect into clouds and then change to liquid water in the form of rain. Your local weather person will always talk about the chance for precipitation. They mean the chance it will rain or fog or cause dew to form on your car. In the case of water in the air the process can also be called condensation, which we talked about in a previous lesson.
For the purposes of chemistry classes we do not usually talk about precipitation happening in the form of rain. Instead we often talk about two different aqueous solutions coming together in one container and how those two solutions may cause solid compounds or molecules to form. This solid compound or molecule that forms can also be referred to as the precipitant. An example of how this precipitant is written in a balanced chemical equation is given below:
6 KOH(aq) + Fe2(SO4)3(aq) –—-> 2 Fe(OH)3(s) + 3 K2SO4(aq)
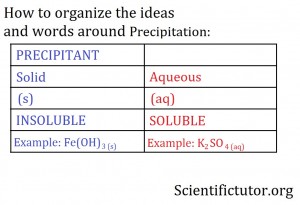
The example means that one solution containing KOH in one cup and another solution containing Fe2(SO4)3 in a completely separate cup are poured together into a container to create the solid precipitate Fe(OH)3 and an aqueous solution of K2SO4 . In this section, you want to be able use definitions to describe the different parts and states in the chemicals equations. The words you will be using to describe the chemical equations are PRECIPITANT, SOLID, INSOLUBLE, AQUEOUS, and SOLUBLE. I have arranged how I want you to think about these words in a small table below. All the words or symbols in BLUE mean the same and all the words or symbols in RED mean the opposite of the blue.
Examples: Determine which chemicals are the PRECIPITANT which are INSOLUBLE, and which are SOLUBLE in the balanced chemical equations below. VIDEO Precipitant Examples 1.
2 Na3PO4(aq) + 3 CaCl2(aq) ——> Ca3(PO4)2(s) + 6 NaCl(aq)
Answer: The PRECIPITANT and INSOLUBLE chemical is Ca3(PO4)2 . The SOLUBLE chemicals are NaCl and Na3PO4 and CaCl2 .
3 MgSO3(aq) + 2 K3PO4(aq) ——> 3 K2(SO3)(aq) + Mg3(PO4)2(s)
Answer: The PRECIPITANT and INSOLUBLE chemical is Mg3(PO4)2 . The SOLUBLE chemicals are MgSO3 and K3PO4 and K2(SO3) .
PRACTICE PROBLEMS: Determine which chemicals are the PRECIPITANT which are INSOLUBLE, and which are SOLUBLE in the balanced chemical equations below. Use the solubility picture if needed.
CaCl2(aq) + Rb2CO3(aq) ——> CaCO3(s) + 2 RbCl(aq)
Answer: The PRECIPITANT and INSOLUBLE chemical is CaCO3 . The SOLUBLE chemicals are CaCl2 and Rb2CO3 and RbCl .
2 CrBr3(aq) + 3 (NH4)2S(aq) ——> 6 NH4Br(aq) + Cr2S3(s)
Answer: The PRECIPITANT and INSOLUBLE chemical is Cr2S3 . The SOLUBLE chemicals are CrBr3 and (NH4)2S and NH4Br .
Sr(OH)2(aq) + 2 HgC2H3O2(aq) ——> 2 HgOH(s) + Sr(C2H3O2)2(aq)
Answer: The PRECIPITANT and INSOLUBLE chemical is HgOH . The SOLUBLE chemicals are Sr(OH)2 and HgC2H3O2 and Sr(C2H3O2)2 .