Scientific Tutor
Chem – Gas Stoichiometry
What is gas stoichiometry?
In this section you are going to put to use a lot of the previous information you have learned in this lesson. You are also going to combine the information from the gas laws lesson with other sections like calculating the molar mass of compounds and combining stoichiometry and molar mass conversions. Make sure you have gone over or know these past sections before you enter this section. One new item that we need to understand in this chapter is how we treat STP conditions when we are doing stoichiometry. If you use the ideal gas law equation at STP conditions and calculate how many liters one mole will produce you come out with 22.4 L. So we can use this as a ratio for gas stoichiometry. I have demonstrated it below.
At STP this is a ratio you can use:
| 22.4 L |
| 1 mol |
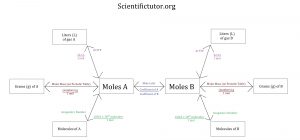
We can now fit this into our new conversions map for gas stoichiometry and use it in the gas stoichiometry problems below.
VIDEO Gas Stoichiometry Demonstrated Example 1: At STP 3 L of H2 gas can make how many moles of NH3?
N2(g) + 3 H2(g) —-> 2 NH3(g)
Step 1:
What information does the question supply us with?
Answer: 3 L H2
Step 2:
What units does the question ask?
Answer: mol NH3
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 2 arrows when we go from Liters of A —> moles of A —> moles of B. 2 arrows = 2 conversions
Step 4:
How do we set up the problem?
Answer:
| 3 L of H2 | mol NH3 | ||
| 1 |
Step 5:
What is the first conversion?
Answer: STP ratio (liters to mole ratio)
Step 6:
How do I put that in?
Answer: units first, set up the units that need to cancel out (in red)
| 3 L of H2 | mol | mol NH3 | |
| L |
Step 7:
What is the next step?
Answer: Fill in the numbers and cross out units
| 3 L of H2 | 1 mol | mol NH3 | |
| 22.4 L |
Step 8:
Simplifiy
| 3 H2 | 1 mol | mol NH3 | |
| 22.4 |
Step 9:
What is the next conversion?
Answer: mole to mole ratio (coefficient ratio)
Step 10:
How do I set it up?
Answer: units first, set up the units that you need to cancel out (in red)
| 3 H2 | 1 mol | NH3 = | mol NH3 |
| 22.4 | H2 |
Step 11:
What is the next step?
Answer: Fill in the numbers and cross out units
| 3 H2 | 1 mol | 2 NH3 = | mol NH3 |
| 22.4 | 3 H2 |
Step 12:
Simplify
| 3 | 1 mol | 2 NH3 = | mol NH3 |
| 22.4 | 3 |
Step 13:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case mol and NH3
| 3 | 1 mol | 2 NH3 = | mol NH3 |
| 22.4 | 3 |
Step 14:
How do I do the calculations?
Answer: (3 * 2) / (22.4 * 3) = 0.89
| 3 | 1 mol | 2 NH3 = | 0.89 mol NH3 |
| 22.4 | 3 |
COMPLETE ANSWER: 0.089 mol NH3
VIDEO Gas Stoichiometry Demonstrated Example 2: How many Liters of O2 gas at STP can be made from a sample of 18 grams of H2O? You will need a periodic table to help solve this problem.
2 C6H11OH(l) + 17 O2(g) ——> 12 CO2(g) + 12 H2O(g)
Step 1:
What information does the question supply us with?
Answer: 18 g H2O
Step 2:
What units does the question ask?
Answer: L O2
Step 3:
How many conversions must we do?
Answer: Look at the conversion map. We pass through 3 arrows when we go from Grams of A —> moles of A —> moles of B —> Liters of B. 3 arrows = 3 conversions
Step 4:
How do we set up the problem?
Answer:
| 18 g H2O | L O2 | |||
| 1 |
Step 5:
What is the first conversion?
Answer: molar mass (grams to mole ratio) of H2O found on the periodic table
Step 6:
How do I put that in?
Answer: units first, set up the units that need to cancel out (in red)
| 18 g H2O | mol | L O2 | ||
| g |
Step 7:
What is the next step?
Answer: Fill in the numbers and cross out units
| 18 g H2O | 1 mol | L O2 | ||
| 18 g |
Step 8:
Simplify
| 18 H2O | 1 mol | L O2 | ||
| 18 |
Step 9:
What is the next conversion?
Answer: mole to mole ratio (coefficient ratio)
Step 10:
How do I set it up?
Answer: units first, set up the units that you need to cancel out (in red)
| 18 H2O | 1 mol | O2 | L O2 | |
| 18 | H2O |
Step 11:
What is the next step?
Answer: Fill in the numbers and cross out units
| 18 H2O | 1 mol | 17 O2 | L O2 | |
| 18 | 12 H2O |
Step 12:
Simplify
| 18 | 1 mol | 17 O2 | L O2 | |
| 18 | 12 |
Step 13:
What is the next conversion?
Answer: STP ratio (liters to mole ratio)
Step 14:
How do I put that in?
Answer: units first, set up the units that need to cancel out (in red)
| 18 | 1 mol | 17 O2 | L = | L O2 |
| 18 | 12 | mol |
Step 15:
What is the next step?
Answer: Fill in the numbers and cross out units
| 18 | 1 mol | 17 O2 | 22.4 L = | L O2 |
| 18 | 12 | 1 mol |
Step 16:
Simplify
| 18 | 1 | 17 O2 | 22.4 L = | L O2 |
| 18 | 12 | 1 |
Step 17:
How do I know I am done with conversions?
Answer: The only units left are the units that match the answer. In this case L and O2
| 18 | 1 mol | 17 O2 | 22.4 L = | L O2 |
| 18 | 12 | 1 |
Step 18:
How do I do the calculations?
Answer: (18 * 17 * 22.4) / (18 * 12) = 31.7
| 18 | 1 mol | 17 O2 | 22.4 L = | 31.7 L O2 |
| 18 | 12 | 1 |
COMPLETE ANSWER: 31.7 L O2
PRACTICE PROBLEMS: Solve these gas stoichiometry problems. Don’t forget to use the periodic table and the conversion map when you need it.
At STP 7 L of O2 gas can make how many moles of MgO?
2 Mg(s) + O2(g) —> 2 MgO(s)
Answer: 0.625 mol MgO
How many Liters of Br2 gas at STP can be made from a sample of 26 grams of AlBr3?
2 AlBr3(s) —> 2 Al(s) + 3 Br2(g)
Answer: 3.27 L Br2
At STP 0.3 L of CO2 requires how many molecules of H2O to completely react?
CH4(g) + 2 O2(g) ——> CO2(g) + 2 H2O(g)
Answer: 1.61 * 1022 molecules H2O
How many Liters of H2 gas at STP are required to completely react with a sample of 4.5 Liters of O2?
6 C(s) + 6 H2(g) + 3 O2(g) ——> C6H12O6(s)
Answer: 9 L O2