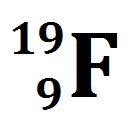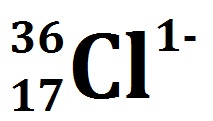Scientific Tutor
Chem – Atomic Notation Part 1
What sections should I know before attempting to learn this section?
—> Neutrons
—> Isotopes
—> Ions
What is atomic notation?
DO NOT USE THE PERIODIC TABLE as a reference in this section. When given the atomic notation you should have all the information you need and should not have to look elsewhere. So far we have been looking at the different elements on the periodic table and we have noticed that the atomic number is on top the symbol is in the middle and the mass is on the bottom. This is what they usually call the periodic notation.
However, we have also begun to explore an alternative way to write out that information. This alternative way is called the atomic or nuclear notation. In the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification.
VIDEO Atomic Notation Demonstrated Example 1: If you have the atomic notation of Fluorine below how many protons, electrons, and neutrons would it have?
The 19 at the top left is the atomic mass.So what do we know about the Fluorine atom from this depiction?
The 9 at the bottom left is the proton number.
NO CHARGE at the top right.
So what are some of the questions can we answer about the Chlorine ion from this depiction?
How many protons does it have? 9 protons
How many electrons does it have? 9 – (0) = 9 electrons
How many neutrons does it have? 19 – 9 = 10 neutrons
VIDEO Atomic Notation Demonstrated Example 2: If you have the atomic notation of Chlorine below how many protons, electrons, and neutrons would it have?
So what do we know about the Chlorine ion from this depiction?
The 36 at the top left is the atomic mass.
The 17 at the bottom left is the proton number.
The (1-) at the top right is the charge.
So what are some of the questions can we answer about the Chlorine ion from this depiction?
How many protons does it have? 17 protons
How many electrons does it have? 17 – (-1) = 18 electrons
How many neutrons does it have? 36 – 17 = 19 neutrons
VIDEO Atomic Notation Demonstrated Example 3: If you have the atomic notation of Beryllium below how many protons, electrons, and neutrons would it have?
The 9 at the top left is the atomic mass.So what do we know about the Beryllium ion from this depiction?
The 4 at the bottom left is the proton number (atomic number).
The 2+ at the top right is the charge.
So what are some of the questions can we answer about the Beryllium ion from this depiction?
How many protons does it have? 4 protons
How many electrons does it have? 4 – (+2) = 2 electrons
How many neutrons does it have? 9 – 4 = 5 neutrons
PRACTICE PROBLEMS: Answer the questions below.
How many protons does the element above have? 26 protons
How many electrons does the element above have? 26 – (3) = 23 electrons
How many neutrons does the element above have? 56 – 26 = 30 neutrons
How many protons does the element above have? 19 protons
How many electrons does the element above have? 19 – (1) = 18 electrons
How many neutrons does the element above have? 39 – 19 = 20 neutrons
How many protons does the element above have? 8 protons
How many electrons does the element above have? 8 – (-2) = 10 electrons
How many neutrons does the element above have? 16 – 8 = 8 neutrons
How many protons does the element above have? 35 protons
How many electrons does the element above have? 35 – (-1) = 36 electrons
How many neutrons does the element above have? 82 – 35 = 47 neutrons
How many protons does the element above have? 10 protons
How many electrons does the element above have? 10 – (0) = 10 electrons
How many neutrons does the element above have? 20 – 10 = 10 neutrons