Scientific Tutor
Chem – Ions
What sections should I know before attempting to learn this section?
What are ions?
Ions are charged atoms of an element. You can create a charged atom of an element by a loss or gain electrons. This is because protons are at the center of the atom and therefore cannot be lost or gained. Since electrons are on the outside of atoms they can be lost to gained. So really ions are all about the electrons. Electrons are negatively charged. Therefore, when atoms gain an electron they become a negative ion (anion) or negatively charge and when they lose an electron they become a positive ion (cation) or positively charge.
Adding ONE electron to an atom = -1 charge
Adding TWO electrons to an atom = -2 charge
Taking away ONE electron from an atom = +1 charge
Taking away TWO electrons from an atom = +2 charge
How do we represent ions with elemental symbols?
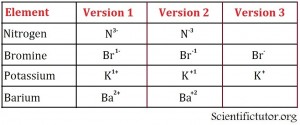
Ions are represented next to elemental symbols by a positive or negative charge and possibly a number on the upper right side of the elemental symbol. Examples of different ways to write the elements are in the pictures below.
How are the ions displayed on the periodic table?
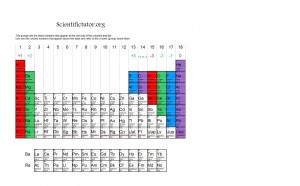
In general, ions are found according to the columns of the periodic table. On most periodic tables, they label the columns 1 through 18 across the top and call them groups. Group 1 starts with Hydrogen and then Lithium. Group 2 starts with Beryllium and then Magnesium. At the end, we have group 18, which starts with Helium and Neon. For now we will only be focusing on group 1,2, 13, 14, 15, 16, and 17. The groups and the ions are best viewed on this periodic table picture below.
Examples: Give the ion of the element and state how many electrons it gained or lost. Use the ion periodic table. VIDEO Ion Examples 1.
| Element | Ion (charged atom) | Electrons |
| Nitrogen | N3- | Gained 3 |
| Bromine | Br1- | Gained 1 |
| Potassium | K1+ | Lost 1 |
| Barium | Ba2+ | Lost 2 |
PRACTICE PROBLEMS: Give the ion of the element and state how many electrons it gained or lost. Make sure you have the ion periodic table link open when answering these questions.
| Element | Ion (charged atom) | Electrons |
| Calcium | Ca2+ | Lost 2 |
| Arsenic | As3- | Gained 3 |
| Fluorine | F1- | Gained 1 |
| Boron | B3+ | Lost 3 |
| Cesium | Cs+ | Lost 1 |
| Sulfur | S2- | Gained 2 |