Scientific Tutor
Chem – College: Molecular Forces and States of Matter
How do molecular forces affect states of matter?
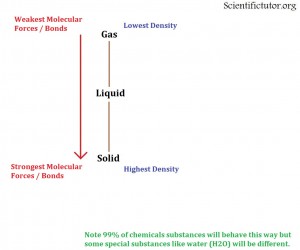
When you are comparing two chemicals with very similar molar mass and you are trying to determine any possible difference in state between them then you have to move on to the types of molecular forces holding them together. We can recall our previous section on covalent and ionic bonds explained INTRAMOLECULAR forces or bonds and our previous section on Intermolecular forces from Lewis Structures explained INTERMOLECULAR forces or bonds. It turns out that you can use these forces to make predictions as to what the density or what the state of matter is. In general the STRONGER the forces between atoms or molecules the MORE DENSE the chemical and the MORE LIKELY it is to be a SOLID. The WEAKER the forces between two atoms or molecules the LESS DENSE the chemical and the MORE LIKELY it is to be a GAS. This relationship is displayed in the picture I have created below.
Now that we know the relationship between the molecular forces and the states of matter we also have to be told about which molecular forces are stronger than others. The strength of these molecular forces or bonds is in order below:
| Forces/Bonds | Type | |
| Strongest | Covalent | INTRAMOLECULAR |
| Metallic | INTRAMOLECULAR | |
| Ionic | INTRAMOLECULAR | |
| Hydrogen | INTERMOLECULAR | |
| Dipole-Dipole | INTERMOLECULAR | |
| Weakest | London Dispersion | INTERMOLECULAR |
As far as INTRAMOLECULAR forces. If you only know that a substance has either covalent bonds, ionic bonds, or metallic bonds and you know nothing else about it you can make a prediction that the unknown substance will be very dense and will most likely be a solid in the conditions at the surface of the Earth.
Examples: Answer the questions below using the intramolecular and intermolecular forces table above.
VIDEO Molecular Forces and States of Matter Explanation for Example 1
A reaction with an unknown substance allowed us to find that the unknown substance had only covalent bonds. Is the unknown substance more likely to be a liquid or is it more likely to be a solid?
Answer: solid
VIDEO Molecular Forces and States of Matter Explanation for Example 2
A large amount of the two chemicals H2S and O2 are forced in to the same chamber. We see one existing in the gas state and one in the liquid state with no chemical reaction between them. What state is each chemical in and give your reasoning as to why?
Answer: H2S is in the liquid state because it has Dipole-Dipole forces. O2 is the gas state because it has London-Dispersion forces.
VIDEO Molecular Forces and States of Matter Explanation for Example 3
C4 and LiF are each tested to see how dense they are. Before the test take place predict which one will be less dense.
Answer: Because C4 has covalent forces and LiF has ionic forces, LiF is less dense.
VIDEO Molecular Forces and States of Matter Explanation for Example 4
What can we predict about the chemical forces in Earth’s atmosphere?
Answer: Earth’s atmosphere is in a gas state. Therefore, it is most likely held together by London Dispersion forces.
PRACTICE PROBLEMS: Answer the questions below using the intramolecular and intermolecular forces table above.
If you have the two chemicals of NaCl and NHF2 in the same container and one appears to be a gas and the other a liquid? Which one is the gas?
Answer: NHF2 will be the liquid because it has hydrogen bonding and those bonds are weaker than the ionic bonding of NaCl.
SiH4 and PH3 are placed in the same container. When I look at the container it appears one of the chemicals is liquid and one of the chemicals is solid. Which one is most likely to be the liquid and why?
Answer: SiH4 is most likely to be the liquid because it has weaker forces (London-Dispersion).
If you have the two solid chemicals of H2O and CH4 in the same container and you raise the temperature, which one will melt first and why?
Answer: The CH4 because it has London-Dispersion forces which are weaker than the hydrogen bonding of H2O.
A group of astronomers discover a new planet and by looking at it they can tell that the planet is composed of mainly three chemical compounds, Al3Fe2, Br2 and SeCl2. If there is life on this planet what chemical would it breath, what chemical would it swim in, and what chemical would it stand on? Explain your reasoning.
Answer: It would breathe Br2 because it is held together by London-Dispersion forces. It would swim in SeCl2 because it is held together by Dipole-Dipole forces. It would stand on Al3Fe2 because it is held together by metallic bonds.