Scientific Tutor
Chem – College: Molar Mass and States of Matter
How does molar mass affect the state of matter?
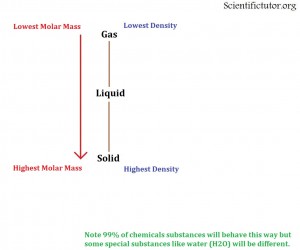
The molar mass of an element or compound has a lot to do with determining its possible state of matter (solid, liquid, gas) generally as the molar mass of a substance increases then it gets more and more dense. As the density increases it is more likely to be in the solid state. Therefore;
As the molar mass of a chemical INCREASES it is more likely to be the MOST dense state possible (usually SOLID).
As the molar mass of a chemical DECREASES it is more likely to be the LEAST dense state possible (usually GAS).
Examples: Answer the questions below. Molar mass versus states of matter examples below.
From the compounds below which one is most likely to be a solid?
NaCl, LiF, RbI, KBr
Answer: RbI
Out of the molecules below which one is most likely to be a gas?
F2, Cl2, Br2, I2
Answer: F2
PRACTICE PROBLEMS: Answer the questions below.
From the compounds below which one is most likely to be a gas?
H2S, H2O, H2Se, H2Te
Answer: H2O
Out of the molecules below which one is most likely to be a Solid?
Ar, Xe, Ne, He
Answer: Xe
If you have a 4 separate containers of each chemical below and 3 of them are solids and one is a liquid, which one is most likely to be the liquid?
CsI, RbBr, LiF, KCl
Answer: LiF
Which chemical below will become a gas first?
NH3, PF3, AsBr3, PI3
Answer: NH3