Scientific Tutor
Chem – Definitions of Transition Between States of Matter
How do we cause things to go between different states of matter?
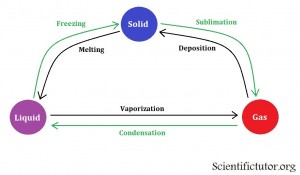
The previous two sections in this lesson suggested how you could change conditions like temperature and pressure to achieve the state of matter that you want from a chemical. However, this section will complete that thought and show you how it is commonly displaced in a chemistry class. The method we use to display these changes in state versus the conditions is called a phase diagram. Before we start analyzing a phase diagram we must first understand the definitions that are used to talk about actions on the phase diagram. We want to know all the words to describe each change in state. All those words are contained in the picture below.
This picture above suggests that it is possible to go directly from solid to gas state without going through the liquid state. The best example of this situation is dry ice. Click the link to see it.
Examples: Give the name for each specific change in state.
| From liquid to solid | freezing |
| From gas to liquid | condensation |
| From liquid to gas | vaporization |
PRACTICE PROBLEMS: Give the name for each specific change in state.
| From solid to liquid | melting |
| From gas to solid | deposition |
| From solid to gas | sublimation |