Scientific Tutor
Chem – Pressure
What is pressure?
The scientific definition of pressure is force divided by area. Again this definition is not that great for giving you an idea of how to think about pressure. Luckily humans have built in experience with pressure so I will use that as an example. We measure the pain we feel in terms of pressure. The higher the pressure the more painful the lower the pressure the less painful. So if you put your whole hand on a table and push down with the weight of your body it is low to zero pain on your hand (low pressure). However, if you put just the tip of one of your fingers down on the table and push down with the weight of your body then you feel lots of pain in your finger (high pressure). What changed in that situation? You had the same weight or mass pressing down, but the amount of area of your body that was in contact with the table was different. When you had a large amount of area touching the table (whole hand) it was not very painful but when you had a tiny area touching the table (one finger) it was very painful. This is the demonstration of force divided by area.
How does the pressure affect the state of a chemical?
To think of pressure in terms of chemistry we want to ask ourselves again what we are doing to the molecules. If we increase the pressure on two molecules (like in a gas state) we are squeezing them together. In other words:
The HIGHER the pressure on a chemical the CLOSER the molecules are pushed together.
The LOWER the pressure on a chemical the FURTHER the molecule are pushed apart.
Therefore, based on our knowledge from the molecules and states of matter section we can suggest how the state of matter of a chemical will be affected by pressure.
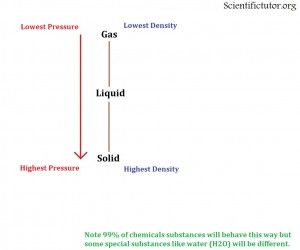
If we constantly drive the PRESSURE HIGHER then that creates LESS SPACE between the molecules and they will eventually be in a SOLID STATE.
If we constantly drive the PRESSURE LOWER then that creates MORE SPACE between the molecules and they will eventually be in a GAS STATE.
Below I have created a simple picture to describe this situation.