Scientific Tutor
Chem – College: Molecular Shapes and Bond Angle Continued
What are the more advanced molecular shapes (molecular geometry)?
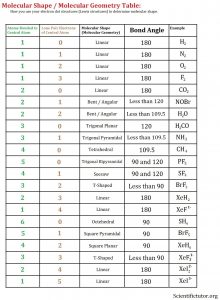
Before taking on this section, make sure you have gone over the completing the octet college section. Those molecular shapes and bond angle beyond Tetrahedral (4 bonds – or electron clouds) are the more advanced shapes. They include shapes like triginal bipyrimidal and octahedral but only make sense if you have explored the electron dot structures (Lewis structures) beyond the octet of 8. You identify these molecular shapes and bond angles from their electron dot structures by counting both the atoms bonded to the central atom and the lone pair electrons of the central atom. Use the complete molecular shape (molecular geometry) table below to answer the examples and practice problems.
Examples: Use the complete molecular shape (molecular geometry) table along with the electron dot structure (Lewis structure) to determine the molecular shape (molecular geometry). VIDEO Molecular Shape from Lewis Structure Examples 2.
(pic SF6)
Atoms bonded to central atom: 6
Lone pair electrons of central atom: 0 pairs
Bond Angle of SF6 (Answer): 90 degrees
Molecular Shape of SF6 (Answer): OCTEHEDRAL
(pic PH5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 1 pair (2 electrons total)
Bond Angle of PH5 (Answer): 90 degrees
Molecular Shape of PH5 (Answer): SQUARE PYRAMIDAL
(pic AsBr3H2)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 0 pairs
Bond Angle of AsBr3H2 (Answer): 90 and 120 degrees
Molecular Shape of AsCl3H2 (Answer): TRIGONAL BIPYRIMIDAL
(pic BrH3)
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 2 pairs (4 electrons total)
Bond Angle of BrH3 (Answer): Less than 90 degrees
Molecular Shape of BrH3 (Answer): T-SHAPED
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) to determine the molecular shape (molecular geometry). You might need the complete molecular shape (molecular geometry) table.
(pic SeBr6)
Atoms bonded to central atom: 6
Lone pair electrons of central atom: 0 pairs
Bond Angle of SeBr6 (Answer): 90 degrees
Molecular Shape of SeBr6 (Answer): OCTAHEDRAL
(PBr5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 0 pairs
Bond Angle of PBr5 (Answer): 120 and 90 degrees
Molecular Shape of PBr5 (Answer): TRIGONAL BIPYRAMIDAL
(pic KrH2)
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 3 pair
Bond Angle of KrH2 (Answer): 180 degrees
Molecular Shape of KrH2 (Answer): LINEAR
(BrH5)
Atoms bonded to central atom: 5
Lone pair electrons of central atom: 1 pairs
Bond Angle of BrH5 (Answer): 90 degrees
Molecular Shape of BrH5 (Answer): SQUARE PYRAMIDAL
(KrH31-)
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 3 pair
Bond Angle of KrH31- (Answer): Less than 90 degrees
Molecular Shape KrH31- (Answer): T-SHAPED
(XeF4)
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 2 pairs
Bond Angle of XeF4 (Answer): 90 degrees
Molecular Shape of XeF4 (Answer): SQUARE PLANAR