Scientific Tutor
Chem – Molecular Shape (Molecular Geometry)
How do electron dot structures (Lewis structures) relate to the molecular (3 dimensional) shapes?
Many molecules but not all you create by electron dot structures (Lewis structures) exist not a flat two dimensional molecules like you drew them on a piece of paper but occupy 3 dimensions. This is driven by the electrons in the bonds and lone pairs of the central atom repelling each other and trying to maintain the furthest distance apart as possible. The theory of electrons repelling each other to create the shape of the molecule is called the VSEPR (Valence Shell Electron Pair Repulsion) model.
How do you determine the molecular shapes (molecular geometry) from the electron dot structure (Lewis structure)?
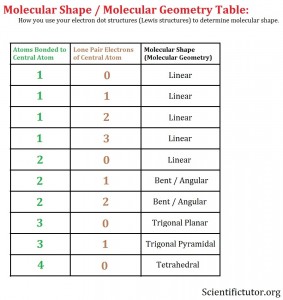
What you are going to focus on to determine the molecular shape of a molecule is the central atom from your electron dot structure. You are going to ask the question, HOW MANY ATOMS ARE ATTACHED TO THE CENTRAL ATOM through bonds AND HOW MANY LONE PAIRS EXIST AROUND ONLY THE CENTRAL ATOM? After that you can refer to the molecular shape table below:
Once you add up the number of atoms bonded to the central atom and the number of lone pairs electrons around the central atom as two distinct and separate numbers then you will get you look over at the molecular shape column and get your answer.
Examples: Use the electron dot structure (Lewis structure) and the molecular shape table to determine the molecular shape (molecular geometry). VIDEO Molecular Shape from Lewis Structure Examples 1.
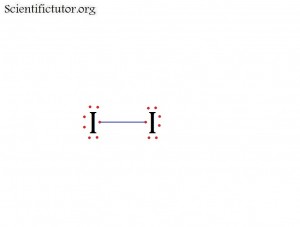
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 3 pairs (6 electrons total)
Molecular Shape of I2 (Answer): LINEAR
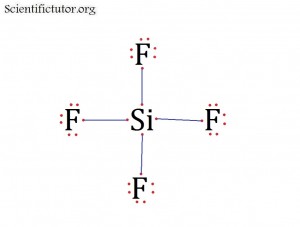
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Molecular Shape of SiF4 (Answer): TETRAHEDRAL
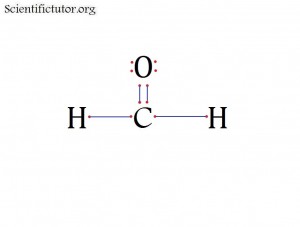
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Molecular Shape of H2CO (Answer): TRIGONAL PLANAR
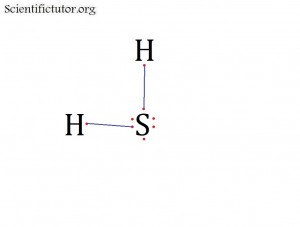
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs (4 electrons total)
Molecular Shape of SH2 (Answer): BENT
PRACTICE PROBLEMS: Use the electron dot structure (Lewis structure) and the molecular shape table to determine the molecular shape (molecular geometry).
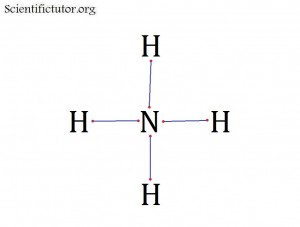
Atoms bonded to central atom: 4
Lone pair electrons of central atom: 0 pairs
Molecular Shape of NH4+ (Answer): TETRAHEDRAL
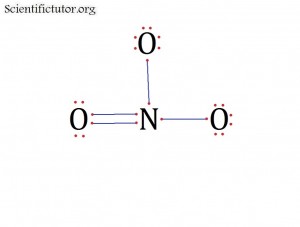
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 0 pairs
Molecular Shape of NO3– (Answer): TRIGONAL PLANAR
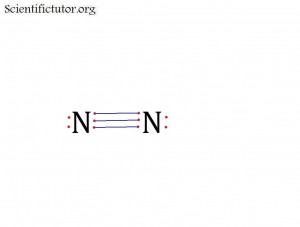
Atoms bonded to central atom: 1
Lone pair electrons of central atom: 1 pair
Molecular Shape of N2 (Answer): LINEAR
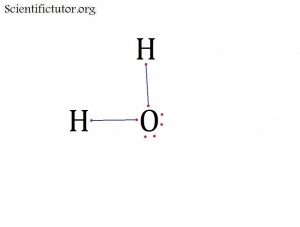
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 2 pairs
Molecular Shape of H2O (Answer): BENT
Atoms bonded to central atom: 3
Lone pair electrons of central atom: 1 pair
Molecular Shape of PCl3 (Answer): TRIGONAL PYRAMIDAL
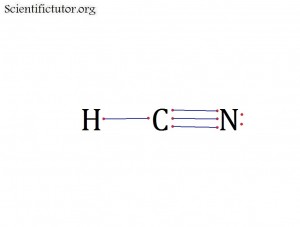
Atoms bonded to central atom: 2
Lone pair electrons of central atom: 0 pairs
Molecular Shape of HCN (Answer): LINEAR
NT