Scientific Tutor
Chem – Types of Chemical Equations
What are the different types of chemical equations?
With many chemical equations you can break them down by the type of chemical equation. This helps you to predict how different chemicals will react when mixing them. The different types are below in bold with clear examples. Look at them and see what kind of patterns you can recognize. Use the periodic table or ion periodic table link if you need it.
SYNTHESIS (also known as combination):
Definition: Two or more chemicals on the reactants become a single chemical on the products.
Example 1: Na+(aq) + Cl–(aq) ——> NaCl(aq)
Example 2: Ca(s) + Br2(g) ——> CaBr2(s)
Example 3: 6 C(s) + 6 H2(g) + 3 O2(g) ——> C6H12O6(s)
Generic Example: A + B ——> AB
DECOMPOSITION:
Definition: One chemical on the reactants becoming two or more on the products.
Example 1: LiOH(aq) ——> Li+(aq) + OH–(aq)
Example 2: 2 K2O(s) ——> 4 K(s) + O2(g)
Example 3: 2 HCN(aq) ——> H2(g) + 2 C(s) + N2(g)
Generic Example: AB ——> A + B
SINGLE REPLACEMENT (also known as single displacement):
Definition: One set of chemical partners swap as they go from reactants to products.
Example 1: Na(s) + LiF(aq) ——> Li(s) + NaF(aq)
Example 2: 2 Fe(NO3)3(aq) + 3 Be(s) ——> 3 Be(NO3)2(s) + 2 Fe(s)
Example 3: 3 (NH4)2(CO3)(aq) + 2 PO43-(aq) ——> 2 (NH4)3(PO4)(aq) + 3 CO32-(aq)
Generic Example: AB + C ——> AC + B
DOUBLE REPLACEMENT (also known as double displacement):
Definition: Two sets of chemical partners swap as they go form reactants to products.
Example 1: KBr(aq) + LiCl(aq) ——> KCl(aq) + LiBr(aq)
Example 2: 3 MgS(aq) + 2 Cs3N(aq) ——> 3 Cs2S(aq) + Mg3N2(s)
Example 3: 2 Al(OH)3(aq) + 3 CaSO4(aq) ——> Al2(SO4)3(aq) + 3 Ca(OH)2(aq)
Generic Example: AB + CD ——> AD + CB
COMBUSTION:
Definition: A carbon compound and oxygen combined to become CO2 and H2O
Example 1: CH4(g) + 2 O2(g) ——> CO2(g) + 2 H2O(g)
Example 2: C6H12O6(s) + 6 O2(g) ——> 6 CO2(g) + 6 H2O(g)
Example 3: CH3COOH(aq) + 4 O2(g) ——> 2 CO2(g) + 2 H2O(g)
Generic Example: carbon compound + O2(g) ——> CO2(g) + H2O(g)
Why kinds of questions can they ask you about the types of chemical equations?
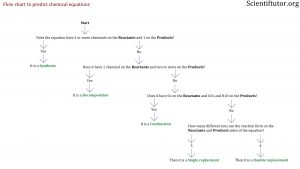
The first kinds of questions you want to be able to answer with the above information is if you are given a complete chemical equation. You want to be able to identify which one of the 5 types of chemical equations it is. To do that the best way is to ask questions about the chemical equations in front of you. Below is a picture of the flow chart of the questions and information that allow you to either confirm or deny the different types of chemical equations. Look to the demonstrated example videos and text to understand how to use the flow chart.
VIDEO Types of Chemical Equations Demonstrated Example 1:Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
Rb2S(s) ——> 2 Rb+(aq) + S2-(aq)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If Yes: Then it is a DECOMPOSTION reaction
VIDEO Types of Chemical Equations Demonstrated Example 2: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
2 Ag3P(s) + 3 V(s) ——> V3P2(s) + 6 Ag(s)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If No: Then it is NOT a combustion
Step 4:
Answer: Follow the NO arrows on the flow chart and ask the next question.
How many different ions can the reaction form on the reactants and products sides of the equation?
Ag forms a positive ion (that is 1), P forms a negative ion (that is 2), V forms a positive ion (that is 3)
If 3 on each side: Then it is a SINGLE REPLACEMENT
VIDEO Types of Chemical Equations Demonstrated Example 3: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
C3H8(l) + 10 O2(g) ——> 3 CO2(g) + 4 H2O(g)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If Yes: Then it is a combustion reaction
VIDEO Types of Chemical Equations Demonstrated Example 4: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
2 NaNO3(aq) + Mg(C2H3O2)2(aq) ——> Mg(NO3)2(aq) + 2 NaC2H3O2(aq)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If No: Then it is NOT a synthesis
Step 2:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have 1 chemical on the REACTANTS side and 2 or more on the PRODUCTS side?
If No: Then it is NOT a decomposition reaction
Step 3:
Answer: Follow the NO arrows on the flow chart and ask the next question.
Does it have O2 in its REACTANTS and CO2 and H2O in its PRODUCTS?
If No: Then it is NOT a combustion
Step 4:
Answer: Follow the NO arrows on the flow chart and ask the next question.
How many different ions can the reaction form on the reactants and products sides of the equation?
Na forms a positive ion (that is 1), NO3 forms a negative ion (that is 2), Mg forms a positive ion (that is 3), C2H3O2 forms a negative ion (that is 4)
If 4 on each side: Then it is a double replacement
VIDEO Types of Chemical Equations Demonstrated Example 5: Give the type of chemical equation of the reaction below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also.
3 Be(s) + N2(g) ——> Be3N2(s)
Step 1:
How do I start to answer?
Answer: Use the flow chart and ask the first question.
Does it have 1 chemical on the PRODUCTS side and 2 or more on the REACTANTS side?
If Yes: Then it is a synthesis reaction
PRACTICE PROBLEMS: Determine which type of chemical equation each of them is below. Most likely you will need the periodic table link. If you forgot the ions go back to the ions of the periodic table also. Try to answer these questions WITHOUT the flow chart but use it if needed.
| H2SO4(aq) —–> H2(g) + S(s) + 2 O2(g) |
| Answer: Decomposition |
| Ba(OH)2(aq) + 2 HCl(aq) ——> 2 H2O(l) + BaCl2(aq) |
| Answer: Double Replacement |
| N2(g) + 3 H2(g) —-> 2 NH3(g) |
| Answer: Synthesis |
| 6 Ag(s) + Ca3(PO4)2(s) ——> 3 Ca(s) + 2 Ag3PO4(s) |
| Answer: Single Replacement |
| 3 NiF2(s) + 2 (NH4)3P(s) ——> 6 NH4F(aq) + Ni3P2(s) |
| Answer: Double Replacement |
| 2 C4H10(l) + 13 O2(g) ——> 8 CO2(g) + 10 H2O (g) |
| Answer: Combustion |
| 3 Na(s) + CrCl3(s) ——> 3 NaCl(aq) + Cr(s) |
| Answer: Single Replacement |
| 2 MnI3(s) ——> 3 I2(g) + Mn(s) |
| Answer: Decomposition |